Is Mayenite without Clathrated Oxygen an Inorganic Electride?†
This work is partially supported by the National Project for the Development of Key Fundamental Sciences in China (G 1 999 075 305, G 2001 CB 3095), by the National Natural Science Foundation of China (50 121 202, 20 025 309, 10 074 058), by the Foundation of Ministry of Education of China, and by the Foundation of the Chinese Academy of Science. We thank Prof. James L. Dye for helpful discussions.
Graphical Abstract
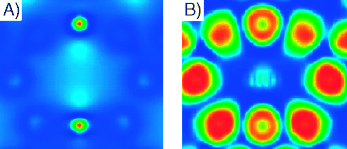
Yes or no? Mayenite without the clathrated oxygen can be classified as an inorganic electride based on combined charge-density (see picture, A) and electron-localization-function (ELF) analysis (B). Ionic chemical bonds are found to form between extra electrons and the positively charged crystal framework in this material.