Photoswitchable Organic Nanoparticles and a Polymer Film Employing Multifunctional Molecules with Enhanced Fluorescence Emission and Bistable Photochromism†
Seon-Jeong Lim
School of Materials Science and Engineering ENG445, Seoul National University, San 56-1, Shillim-dong, Kwanak-ku, Seoul 151-744, Korea, Fax: (+82) 2-886-8331
Search for more papers by this authorByeong-Kwan An
School of Materials Science and Engineering ENG445, Seoul National University, San 56-1, Shillim-dong, Kwanak-ku, Seoul 151-744, Korea, Fax: (+82) 2-886-8331
Search for more papers by this authorSang Don Jung Dr.
Basic Research Laboratory, Electronics and Telecommunications Research Institute (ETRI), 161 Gajeong-dong, Yuseong-gu, Daejeon 305-350, Korea
Search for more papers by this authorMyung-Ae Chung Dr.
Basic Research Laboratory, Electronics and Telecommunications Research Institute (ETRI), 161 Gajeong-dong, Yuseong-gu, Daejeon 305-350, Korea
Search for more papers by this authorSoo Young Park Prof.
School of Materials Science and Engineering ENG445, Seoul National University, San 56-1, Shillim-dong, Kwanak-ku, Seoul 151-744, Korea, Fax: (+82) 2-886-8331
Search for more papers by this authorSeon-Jeong Lim
School of Materials Science and Engineering ENG445, Seoul National University, San 56-1, Shillim-dong, Kwanak-ku, Seoul 151-744, Korea, Fax: (+82) 2-886-8331
Search for more papers by this authorByeong-Kwan An
School of Materials Science and Engineering ENG445, Seoul National University, San 56-1, Shillim-dong, Kwanak-ku, Seoul 151-744, Korea, Fax: (+82) 2-886-8331
Search for more papers by this authorSang Don Jung Dr.
Basic Research Laboratory, Electronics and Telecommunications Research Institute (ETRI), 161 Gajeong-dong, Yuseong-gu, Daejeon 305-350, Korea
Search for more papers by this authorMyung-Ae Chung Dr.
Basic Research Laboratory, Electronics and Telecommunications Research Institute (ETRI), 161 Gajeong-dong, Yuseong-gu, Daejeon 305-350, Korea
Search for more papers by this authorSoo Young Park Prof.
School of Materials Science and Engineering ENG445, Seoul National University, San 56-1, Shillim-dong, Kwanak-ku, Seoul 151-744, Korea, Fax: (+82) 2-886-8331
Search for more papers by this authorThis work was supported in part by CRM-KOSEF and ETRI.
Graphical Abstract
Get turned on: Fluorescent photochromic organic nanoparticles (FPONs) of 1 (see picture) show a strongly enhanced fluorescence emission with increasing concentration and bistable photochromism. High-contrast on/off fluorescence switching has been successfully implemented in size-tuned FPONs of 1 and also in a photo-rewritable polymer film highly loaded with 1.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z461172_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. Fernandez, J.-M. Lehn, Adv. Mater. 1998, 10, 1519–1522.
- 2T. Kawai, T. Sasaki, M. Irie, Chem. Commun. 2001, 711–712.
- 3K. Yagi, C. F. Soong, M. Irie, J. Org. Chem. 2001, 66, 5419–5423.
- 4A. Osuka, D. Fujikane, H. Shinmori, S. Kobatake, M. Irie, J. Org. Chem. 2001, 66, 3913–3923.
- 5T. B. Norsten, N. R. Branda, J. Am. Chem. Soc. 2001, 123, 1784–1785.
- 6B. Chen, M. Wang, Y. Wu, H. Tian, Chem. Commun. 2002, 1060–1061.
- 7T. Kawai, M.-S. Kim, T. Sasaki, M. Irie, Opt. Mater. 2002, 21, 275–278.
- 8M. Irie, T. Fukaminato, T. Sasaki, N. Tamai, T. Kawai, Nature 2002, 420, 759–760.
- 9S. Murase, M. Teramoto, H. Furukawa, Y. Miyashita, K. Horie, Macromolecules 2003, 36, 964–966.
- 10M. Irie, Photoreactive Materials for Ultrahigh-Density Optical Memory, Elsevier, Amsterdam, 1994.
- 11J. C. Crano, R. J. Guglielmetti, Organic Photochromic and Thermochromic Compounds, Vol. 1, Plenum, New York, 1999.
- 12S. Nakamura, M. Irie, J. Org. Chem. 1988, 53, 6136–6138.
- 13Y. Nakayama, K. Hayashi, M. Irie, J. Org. Chem. 1990, 55, 2592–2596.
- 14M. Hanazawa, R. Sumiya, Y. Horikawa, M. Irie, J. Chem. Soc. Chem. Commun. 1992, 206–207.
- 15M. Irie, Chem. Rev. 2000, 100, 1685–1716.
- 16J. B. Birks in Photophysics of Aromatic Molecules, Wiley, London, 1970.
- 17J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu, B. Z. Tang, Chem. Commun. 2001, 1740–1741.
- 18H. Murata, Z. H. Kafafi, M. Uchida, Appl. Phys. Lett. 2002, 80, 189–191.
- 19B. K. An, S. K. Kwon, S. D. Jung, S. Y. Park, J. Am. Chem. Soc. 2002, 124, 14 410–14 415.
- 20Preparation of FPONs: All suspensions of FPONs were prepared by the reprecipitation method from THF solution with distilled water. Volume fractions of THF and water were adjusted to 20 and 80 %, respectively. See ref. [19] for details.
- 21Fluorescence quantum yields: The fluorescence quantum yields (ΦF) were relatively calculated using 9,10-diphenylanthracene (DPA) in benzene (see ref. [22]) and in PMMA (see refs. [29,30]) as a standard reference (1×10−3 m, ΦF=83 %). Through this method, the ΦF values of FPONs of 1 a were determined as 3.2, 5.1, 4.1, and 3.4 % (
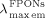 =485, 489, 491, and 493 nm,
=485, 489, 491, and 493 nm, 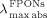 =371, 374, 378, and 382 nm) in the 1 a form, and as 0.20, 0.09, 0.04, and 0.02 % in the PSS with increasing FPON size of 40±10, 125±25, 200±50, and 275±75 nm, respectively. It is reasonably considered that the slight reduction of the ΦF values in 200±50 and 275±75 nm FPONs of 1 a results from the highly increased virtual absorbance values by increased light scattering. In fact, the extent of the apparent absorbance of the FPONs of 1 a at the excitation wavelength, that is at 360 nm, was almost linearly proportional to their suspension concentrations in spite of the inverse proportional relationship between the mean radius and the surface-to-volume ratio of the FPONs.
=371, 374, 378, and 382 nm) in the 1 a form, and as 0.20, 0.09, 0.04, and 0.02 % in the PSS with increasing FPON size of 40±10, 125±25, 200±50, and 275±75 nm, respectively. It is reasonably considered that the slight reduction of the ΦF values in 200±50 and 275±75 nm FPONs of 1 a results from the highly increased virtual absorbance values by increased light scattering. In fact, the extent of the apparent absorbance of the FPONs of 1 a at the excitation wavelength, that is at 360 nm, was almost linearly proportional to their suspension concentrations in spite of the inverse proportional relationship between the mean radius and the surface-to-volume ratio of the FPONs.
- 22I. B. Berlman in Handbook of Fluorescence Spectra of Aromatic Molecules, Academic Press, New York, 1971.
- 23H. Auweter, H. Haberkorn, W. Heckmann, D. Horn, E. Lüddecke, J. Rieger, H. Weiss, Angew. Chem. 1999, 111, 2325–2328;
10.1002/(SICI)1521-3757(19990802)111:15<2325::AID-ANGE2325>3.0.CO;2-Y Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2188–2191.10.1002/(SICI)1521-3773(19990802)38:15<2188::AID-ANIE2188>3.0.CO;2-# CAS PubMed Web of Science® Google Scholar
- 24A. V. Ruban, P. Horton, A. J. Young, J. Photochem. Photobiol. B 1993, 21, 229–234.
- 25L. Dahne, E. Biller, Adv. Mater. 1998, 10, 241–245.
- 26Determination of the conformational population and conversion rate: A 1H NMR spectroscopic study (2×10−4 m of CDCl3 solution, 22 °C, 300 MHz) on the 1 a form showed that the parallel and antiparallel conformations were equally populated in solution, as evident by there only being two singlet resonances at δ=1.98 and 1.91 ppm without any splitting. These signals were assigned as the methyl protons at the 2-positions of the thiophene rings of the 1 a form (see ref. [27]). The conversion rate of about 35 % in the PSS was calculated by the integrated ratio between the singlet peaks of methyl protons in the 1 a form and those in the 1 b form, which newly appeared at δ=2.15 and 2.11 ppm.
- 27M. Irie, K. Sakemura, M. Okinaka, K. Uchida, J. Org. Chem. 1995, 60, 8305–8309.
- 28F. Sun, F. Zhang, F. Zhao, X. Zhou, S. Pu, Chem. Phys. Lett. 2003, 380, 206–212.
- 29G. G. Guilbault, Practical Fluorescence, Marcel Dekker, New York, 1990.
- 30X. Zhang, A. S. Shetty, S. A. Jenekhe, Macromolecules 1999, 32, 7422–7429.





