Design of a Conformation-Sensitive Xenon-Binding Cavity in the Ribose-Binding Protein†
This work was supported by the Director, U.S. Department of Energy, under Contract No. DE-AC03-76F00098, through the Office of Naval Research (MDI-II), and through the Laboratory Directorate Research and Development Program of Lawrence Berkeley National Laboratory. We thank Prof. S. Mowbray for the ribose-binding-protein gene, H. Yokota for helpful advice with cloning, Dr. S. Burley for the pSKB3 plasmid, Dr. D. King for mass spectrometry, Prof. S. Marqusee and Dr. C. Park for providing an isothermal titration calorimeter and guidance with urea melt experiments. S.M.R. acknowledges the National Science Foundation and E.J.R. Lucent Technologies/Bell Laboratories for predoctoral fellowships.
Graphical Abstract
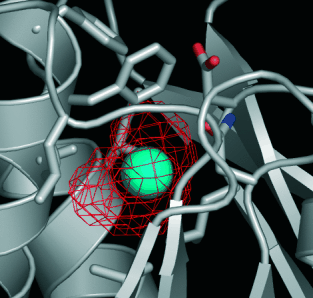
129Xe NMR spectroscopy meets protein engineering: NMR spectroscopy with laser-polarized 129Xe can be used to report protein conformations only if a protein has a conformationally sensitive xenon-binding cavity. However, many proteins lack such reporter sites. A straightforward design process is used to engineer a xenon-binding cavity into the ribose-binding protein (see picture) that allows the 129Xe NMR spectroscopic report of protein conformation and ligand binding.