Amorphous Aluminum Bromide Fluoride (ABF)†
This work was supported by the Deutsche Forschungsgemeinschaft (Ke 489/15-1). We thank Dr. J.-D. Grunwaldt and co-workers from the ETH Zurich for performing the EXAFS measurements.
Graphical Abstract
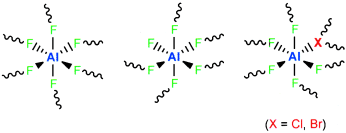
Different octahedral units form the basis of the structures of the very strong Lewis acids aluminum bromide fluoride (ABF) and aluminum chloride fluoride (ACF) (see picture). A structure model for these phases is established in which the octahedra are linked through μ-bridging fluorine atoms and μ3-bridging X atoms (X=Cl, Br). The bridging of three octahedra by a heavy halogen atom explains the results of NMR and EXAFS analysis.