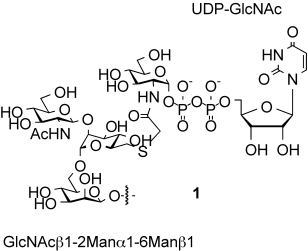
Synthesis of a Bisubstrate-Type Inhibitor of N-Acetylglucosaminyltransferases†
Shinya Hanashima Dr.
RIKEN (The Institute of Physical and Chemical Research), 2–1 Hirosawa, Wako-shi, Saitama 351-0198, Japan, Fax: (+81) 48-462-4680
Search for more papers by this authorShino Manabe Dr.
RIKEN and PRESTO, Japan Science and Technology Agency (JST), Japan
Search for more papers by this authorKei-ichiro Inamori Dr.
Department of Biochemistry, Osaka University Medical School, 2–2 Yamadaoka, Suita, Osaka 565-0871, Japan
Search for more papers by this authorNaoyuki Taniguchi Prof. Dr.
Department of Biochemistry, Osaka University Medical School, 2–2 Yamadaoka, Suita, Osaka 565-0871, Japan
Search for more papers by this authorYukishige Ito Dr.
RIKEN (The Institute of Physical and Chemical Research), 2–1 Hirosawa, Wako-shi, Saitama 351-0198, Japan, Fax: (+81) 48-462-4680
Search for more papers by this authorShinya Hanashima Dr.
RIKEN (The Institute of Physical and Chemical Research), 2–1 Hirosawa, Wako-shi, Saitama 351-0198, Japan, Fax: (+81) 48-462-4680
Search for more papers by this authorShino Manabe Dr.
RIKEN and PRESTO, Japan Science and Technology Agency (JST), Japan
Search for more papers by this authorKei-ichiro Inamori Dr.
Department of Biochemistry, Osaka University Medical School, 2–2 Yamadaoka, Suita, Osaka 565-0871, Japan
Search for more papers by this authorNaoyuki Taniguchi Prof. Dr.
Department of Biochemistry, Osaka University Medical School, 2–2 Yamadaoka, Suita, Osaka 565-0871, Japan
Search for more papers by this authorYukishige Ito Dr.
RIKEN (The Institute of Physical and Chemical Research), 2–1 Hirosawa, Wako-shi, Saitama 351-0198, Japan, Fax: (+81) 48-462-4680
Search for more papers by this authorS.H. is a recipient of the Special Postdoctoral Researcher Program Fellowship in RIKEN. Additional financial support from CREST (JST) is acknowledged. We thank Dr. T. Chihara and his staff for elemental analyses and Dr. H. Koshino and Ms. T. Chijimatsu for NMR measurements. We thank Ms. Akemi Takahashi for her technical assistance.
Graphical Abstract
Transfer interference: Synthesis of the bisubstrate-type N-acetylglucosaminyltransferase (GnT) inhibitor 1 was achieved by a polymer-resin hybrid capture–release strategy for the construction of the acceptor component. One-pot ligation in aqueous media produced the coupling product, and subsequent construction of a diphosphate linkage led to 1. Inhibitory activities toward GnT-V and GnT-IX were evaluated and revealed the potency of 1 toward the latter enzyme.
References
- 1 Handbook of Glycosyltransferases and Related Genes (Eds.: ), Springer, Tokyo, 2002.
- 2
- 2aI. Brockhausen, J. P. Carver, H. Schachter, Biochem. Cell Biol. 1988, 66, 1134–1151;
- 2bR. D. Cummings, I. S. Trowbridge, S. Kornfeld, J. Biol. Chem. 1982, 257, 13 421–13 427.
- 3K. Inamori, T. Endo, Y. Ide, S. Fujii, J. Gu, K. Honke, N. Taniguchi, J. Biol. Chem. 2003, 278, 43 102–43 109.
- 4K. Inamori, T. Endo, J. Gu, I. Matsuo, Y. Ito, S. Fujii, H. Iwasaki, H. Narimatsu, E. Miyoshi, K. Honke, N. Taniguchi, J. Biol. Chem. 2004, 279, 2337–2340.
- 5J. W. Dennis, M. Granovsky, C. E. Warren, Biochim. Biophys. Acta 1999, 1473, 21–34.
- 6aJ. W. Dennis, S. Laferte, C. Waghorne, M. L. Breitman, R. S. Kerbel, Science 1987, 236, 582–585;
- 6bS. Ihara, E. Miyoshi, J. Ko, K. Murata, S. Nakahara, K. Honke, R. B. Dickson, C.-Y. Lin, N. Taniguchi, J. Biol. Chem. 2002, 277, 16 960–16 967;
- 6cM. Demetriou, I. R. Nabi, M. Coppolino, S. Dedhar, J. W. Dennis, J. Cell Biol. 1995, 130, 383–392;
- 6dH.-B. Guo, I. Lee, M. Kamar, S. K. Akiyama, M. Pierce, Cancer Res. 2002, 62, 6837–6845;
- 6eH.-B. Guo, I. Lee, M. Kamar, M. Pierce, J. Biol. Chem. 2003, 278, 52 412–52 424;
- 6fM. Demetriou, M. Granovsky, S. Quaggin, J. W. Dennis, Nature 2001, 409, 733–739;
- 6gT. Saito, E. Miyoshi, K. Sasai, N. Nakano, H. Eguchi, K. Honke, N. Taniguchi, J. Biol. Chem. 2002, 277, 17 002–17 008.
- 7M. Granovsky, J. Fata, J. Pawling, W. J. Muller, R. Khokha, J. W. Dennis, Nat. Med. 2000, 6, 306–312.
- 8J. W. Dennis, J. Pawling, P. Cheung, E. Partridge, M. Demetriou, Biochim. Biophys. Acta 2002, 1573, 414–422.
- 9
- 9aP.-P. Lu, O. Hindsgaul, H. Li, M. M. Palcic, Carbohydr. Res. 1997, 303, 283–291;
- 9bI. Brockhausen, F. Reck, W. Kuhns, S. Khan, K. L. Matta, E. Meinjohanns, H. Paulsen, R. N. Shah, M. A. Baker, H. Schachter, Glycoconjugate J. 1995, 12, 371–379.
- 10Previous reports on bisubstrate-type glycosyltransferase inhibitors:
- 10aH. Hashimoto, T. Endo, Y. Kajihara, J. Org. Chem. 1997, 62, 1914–1915;
- 10bB. Waldscheck, M. Streiff, W. Notz, W. Kinzy, R. R. Schmidt, Angew. Chem. 2001, 113, 4120–4124;
10.1002/1521-3757(20011105)113:21<4120::AID-ANGE4120>3.0.CO;2-Q Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4007–4011;10.1002/1521-3773(20011105)40:21<4007::AID-ANIE4007>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 10cH. Hinou, X.-L. Sun, Y. Ito, J. Org. Chem. 2003, 68, 5602–5613.
- 11I. Tvaroska, I. Andre, J. P. Carver, J. Am. Chem. Soc. 2000, 122, 8762–8776.
- 12S. H. Tahir, O. Hindsgaul, Can. J. Chem. 1986, 64, 1771–1780.
- 13
- 13aH. Ando, S. Manabe, Y. Nakahara, Y. Ito, J. Am. Chem. Soc. 2001, 123, 3848–3849;
- 13bH. Ando, S. Manabe, Y. Nakahara, Y. Ito, Angew. Chem. 2001, 113, 4861–4864;
Angew. Chem. Int. Ed. 2001, 40, 4725–4728;
10.1002/1521-3773(20011217)40:24<4725::AID-ANIE4725>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 13cY. Ito, S. Manabe, Chem. Eur. J. 2002, 8, 3076–3084;
10.1002/1521-3765(20020715)8:14<3076::AID-CHEM3076>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 13dS. Hanashima, S. Manabe, Y. Ito, Synlett 2003, 979–982.
- 14L. Jian, C. Hartley, T.-H. Chan, Chem. Commun. 1996, 2193–2194.
- 15G. Hodosi, P. Kovác, Carbohydr. Res. 1998, 308, 63–75.
- 16
- 16aP. Konradsson, D. R. Mootoo, R. E. McDevitt, B. Fraser-Reid, J. Chem. Soc. Chem. Commun. 1990, 270–272;
- 16bG. H. Veeneman, S. H. van Leeuwen, J. H. van Boom, Tetrahedron Lett. 1990, 31, 1331–1334.
- 17In this particular case, use of a catalytic amount of TfOH afforded the corresponding orthoester exclusively.
- 18C. A. A. van Boeckel, T. Beetz, Tetrahedron Lett. 1983, 24, 3775–3778.
- 19Hydrogenolysis in EtOH caused 3-O-deacetylation as a side reaction.
- 20Preparation of bis(allyloxy)diisopropylaminophosphine 19: W. Bannwarth, E. Kung, Tetrahedron Lett. 1989, 30, 4219–4222.
- 21M. M. Sim, H. Kondo, C.-H. Wong, J. Am. Chem. Soc. 1993, 115, 2260–2267.
- 22V. Wittmann, C.-H. Wong, J. Org. Chem. 1997, 62, 2144–2147.
- 23Spectroscopic data for 1 a: 1H NMR (500 MHz, D2O with a small amount of dioxane as an internal standard, 40 °C): δ=7.78 (d, 3JH,H=7.8 Hz, 1 H), 5.81 (m, 2 H), 5.37 (dd, 3JH,H=3.0, 3JH,P=7.1 Hz, 1 H), 4.76 (br s, 1 H; anomeric proton of αMan), 4.42 (br s, 1 H; anomeric proton of βGlcNAc; overlapped in HOD signal, confirmed by HMQC spectra) 4.20 (br s, 1 H; anomeric proton of βMan) 4.11–3.22 (m), 3.59 (s, 3 H; methyl ester), 2.96 (dd, 3JH,H=14.6, 1.8 Hz, 1 H), 2.58 (dd, 3JH,H=14.6, 8.3 Hz, 1 H), 2.22 (t, 3JH,H=7.3 Hz, 2 H), 1.90 (s, 3 H), 1.42 (br s, 4 H), 1.15 (br s, 8 H) ppm; MALDI-TOF MS (negative mode, CHCA): m/z: calcd for C47H77N4O34P2S [M−H]−: 1335.36; found: 1335.32.
- 24K. Sasai, Y. Ikeda, T. Fujii, T. Tsuda, N. Taniguchi, Glycobiology 2002, 12, 119–127.
- 25N. Taniguchi, A. Nishikawa, S. Fujii, J. G. Gu, Methods Enzymol. 1989, 179, 397–408.