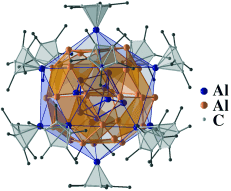
Al50C120H180: A Pseudofullerene Shell of 60 Carbon Atoms and 60 Methyl Groups Protecting a Cluster Core of 50 Aluminum Atoms†
Jean Vollet Dipl.-Chem.
Institut für Anorganische Chemie, Universität Karlsruhe (TH), Engesserstrasse 15, Geb. 30.45, 76128 Karlsruhe, Germany, Fax: (+49) 721-608-4854
Search for more papers by this authorJens R. Hartig Dipl.-Chem.
Institut für Anorganische Chemie, Universität Karlsruhe (TH), Engesserstrasse 15, Geb. 30.45, 76128 Karlsruhe, Germany, Fax: (+49) 721-608-4854
Search for more papers by this authorHansgeorg Schnöckel Prof. Dr.
Institut für Anorganische Chemie, Universität Karlsruhe (TH), Engesserstrasse 15, Geb. 30.45, 76128 Karlsruhe, Germany, Fax: (+49) 721-608-4854
Search for more papers by this authorJean Vollet Dipl.-Chem.
Institut für Anorganische Chemie, Universität Karlsruhe (TH), Engesserstrasse 15, Geb. 30.45, 76128 Karlsruhe, Germany, Fax: (+49) 721-608-4854
Search for more papers by this authorJens R. Hartig Dipl.-Chem.
Institut für Anorganische Chemie, Universität Karlsruhe (TH), Engesserstrasse 15, Geb. 30.45, 76128 Karlsruhe, Germany, Fax: (+49) 721-608-4854
Search for more papers by this authorHansgeorg Schnöckel Prof. Dr.
Institut für Anorganische Chemie, Universität Karlsruhe (TH), Engesserstrasse 15, Geb. 30.45, 76128 Karlsruhe, Germany, Fax: (+49) 721-608-4854
Search for more papers by this authorWe thank the Deutsche Forschungsgemeinschaft, The DFG Research Center for Functional Nanostructures, the Fonds der Chemischen Industrie, and the Bundesministerium für Bildung und Forschung for financial support, especially for a grant to J.R.H.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z53754_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. Schnepf, H. Schnöckel, Angew. Chem. 2002, 114, 4344; Angew. Chem. Int. Ed. 2002, 41, 3533.
- 2A. Purath, C. Dohmeier, A. Ecker, R. Köppe, H. Krautscheid, H. Schnöckel, R. Ahlrichs, C. Stoermer, J. Friedrich, P. Jutzi, J. Am. Chem. Soc. 2000, 122, 6955; C. Dohmeier, C. Robl, M. Tacke, H. Schnöckel, Angew. Chem. 1991, 103, 594; Angew. Chem. Int. Ed. Engl. 1991, 30, 564.
- 3K. Weiß, H. Schnöckel, Anal. Bioanal. Chem. 2003, 377, 1098.
- 4C. Dohmeier, D. Loos, H. Schnöckel, Angew. Chem. 1996, 108, 141;
10.1002/ange.19961080204 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 129.
- 5Crystal data of Mg2Br2(C5Me5)2⋅2 C4H8O: Z=2, Mr=623.09 g mol−1, crystal size: 0.5×0.3×0.2 mm3, monoclinic, space group P2(1)/n, a=8.9706(10), b=13.4564(16), c=12.6868(17) Å, β=94.320(10)°, V=1527.1(3) Å3, ρcalcd=1.355 g cm−3, F(000)=648, λ=0.71073 Å, T=150(2) K, μ(MoKα)=2.72 mm−1, STOE IPDS 2 area detector, 2-circle goniometer, 5073 reflections, 2701 unique (Rint=0.0553), structure solution by direct methods, refinement on F2 (2θmax=51.92°), 2067 unique (2σ), H atoms calculated, 149 parameters, R1(I>2σ(I))=0.0461, wR2(all data)=0.1155, GOF (F2)=1.031, ρ(e)(min/max)=−0.443/0.562 e Å−3; computer programs: SHELXS-97, SHELXL-97, Stoe IPDS-software; unit cell determination: 5073 reflections, corrections: Lorentz, polarisation and numerical absorption corrections, Tmin/Tmax=0.4521/0.7340.
- 6C. Dohmeier, D. Loos, C. Robl, H. Schnöckel, D. Fenske, J. Organomet. Chem. 1993, 448, 5.
- 7Crystal data of 1 (Al50C120H180⋅6 C6H6): Z=2, Mr=3440.29 g mol−1, crystal size: 0.3×0.2×0.15 mm3, orthorhombic, space group Pnnn, a=19.2166(7), b=21.4933(10), c=21.8054(12) Å, V=9006.2(7) Å3, ρcalcd=1.269 g cm−3, F(000)=3604, λ=0.710 73 Å, T=150(2) K, μ(MoKα)=0.297 mm−1, STOE IPDS 2 area detector, 2-circle goniometer, 35 307 reflections, 6838 unique (Rint=0.0958), structure solution by direct methods, refinement on F2 (2θmax=47.32°), 4465 unique (2σ), H atoms calculated, 485 parameters, 3 restraints, R1(I>2σ(I))=0.0742, wR2(all data)=0.2036, GOF (F2)=1.028, restrained GOF=1.028, ρ(e)(min/max)=−0.606/0.819 e Å−3; computer programs: SHELXS-97, SHELXL-97, Stoe IPDS-software; unit cell determination: 19 808 reflections, corrections: Lorentz, polarization, and numerical absorption corrections, Tmin/Tmax=0.9583, 0.9847. CCDC-228316 (Mg2Br2(C5Me5)2⋅2 C4H8O) and 228049 (1) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 8A. Schnepf, G. Stösser, H. Schnöckel, Z. Anorg. Allg. Chem. 2000, 626, 1676.
- 9Q. Yu, A. Purath, A. Donchev, H. Schnöckel, J. Organomet. Chem. 1999, 584, 94.
- 10A snub dodecahedron (Schläfli symbol 34.5) is a geometrical isomer of the C60 fullerene (5.62) and the rhombicosidodecahedron (3.4.5.4). The latter one is evident in the Pd60 shell of the Pd145CO60(PEt3)30 cluster compound. See literature in reference [17].
- 11J. Steiner, G. Stösser, H. Schnöckel, Angew. Chem. 2003, 115, 2016;
10.1002/ange.200250461 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1971.
- 12J. Steiner, G. Stösser, H. Schnöckel, Angew. Chem. 2004, 116, 306;
10.1002/ange.200352472 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 302.
- 13A. Ecker, E. Weckert, H. Schnöckel, Nature 1997, 387, 379.
- 14H. Köhnlein, A. Purath, C. Klemp, E. Baum, I. Krossing, G. Stösser, H. Schnöckel, Inorg. Chem. 2001, 40, 4830.
- 15R. Ahlrichs, S. D. Elliott, Phys. Chem. Chem. Phys. 1999, 1, 13.
- 16M. S. Paquette, L. F. Dahl, J. Am. Chem. Soc. 1980, 102, 6621.
- 17The Al50 compound introduced herein shows that use of microscopic methods such as atomic force microscopy and scanning tunneling microscopy alone would probably have led to inaccurate predictions of structural details. For 1, one would most likely have expected to find an inner shell of 13 instead of just 8 Al atoms (such as in, for example, the Pd13 core of a Pd145 cluster, see N. T. Tran, D. R. Powell, L. F. Dahl, Angew. Chem. 2000, 112, 42 871;
Angew. Chem. Int. Ed. 2000, 39, 412 as well as the Supporting information).
10.1002/(SICI)1521-3773(20000117)39:2<412::AID-ANIE412>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 18K. M. Kadish, R. S. Ruoff, Fullerenes: Chemistry, Physics, and Technology, Wiley-Interscience, New York, 2000, pp. 968; R. E. Dinnebier, O. Gunnarsson, H. Brumm, E. Koch, P. W. Stephens, A. Huq, M. Jansen, Science 2002, 296, 109.
- 19A. Müller, P. Kögerler, A. W. M. Dress, Coord. Chem. Rev. 2001, 222, 193.
- 20J. D. Corbett, Angew. Chem. 2000, 112, 682;
10.1002/(SICI)1521-3757(20000218)112:4<682::AID-ANGE682>3.0.CO;2-3 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 670.10.1002/(SICI)1521-3773(20000218)39:4<670::AID-ANIE670>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 21C. P. Gómez, S. Lidin, Angew. Chem. 2001, 113, 4161;
Angew. Chem. Int. Ed. 2001, 40, 4037.
10.1002/1521-3773(20011105)40:21<4037::AID-ANIE4037>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 22H. Sakurai, S. E. Kooi, A. W. Castleman, J. Cluster Sci. 1999, 10, 493.
- 23For details see the Supporting Information.
- 24W. Uhl, Z. Naturforsch. B 1988, 43, 1113.
- 25W. Hiller, K.-W. Klinkhammer, W. Uhl, J. Wagner, Angew. Chem. 1991, 103, 182; Angew. Chem. Int. Ed. Engl. 1991, 30, 179.
- 26C. Dohmeier, C. Robl, M. Tacke, H. Schnöckel, Angew. Chem. 1991, 103, 594; Angew. Chem. Int. Ed. Engl. 1991, 30, 564.
- 27C. Elschenbroich, Organometallchemie, 4th ed., Teubner, Stuttgart, 2003.
10.1007/978-3-322-99393-9 Google Scholar
- 28A. Simon, Coord. Chem. Rev. 1997, 163, 253.
- 29
- 29aF. X. Kohl, P. Jutzi, Chem. Ber. 1981, 114, 489;
- 29bM. G. Whitesides, D. Feitler, Inorg. Chem. 1976, 15, 466;
- 29cW. A Duff, P. B. Hitchcock, M. F. Lappert, R. G. Taylor, J. A. Segal, J. Organomet. Chem. 1985, 293, 271.
- 30aR. Ahlrichs, M. Bär, M. Häser, H. Horn, C. Kölmel, Chem. Phys. Lett. 1989, 162, 165–169; K. Eichkorn, O. Treutler, H. Öhm, M. Häser, R. Ahlrichs, Chem. Phys. Lett. 1995, 240, 283; K. Eichkorn, O. Treutler, H. Öhm, M. Häser, R. Ahlrichs, Chem. Phys. Lett. 1995, 242, 652; K. Eichkorn, F. Weigend, O. Treutler, R. Ahlrichs, Theor. Chim. Acta 1997, 97, 119; F. Weigend, M. Häser, Theor. Chim. Acta 1997, 97, 331; F. Weigend, M. Häser, H. Patzelt, R. Ahlrichs, Chem. Phys. Lett. 1998, 294, 143;
- 30bGaussian 98 (Revision A.7), M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle, J. A. Pople, Gaussian, Inc., Pittsburgh, PA, 1998;
- 30cJ. B. Foresman, T. A. Keith, K. B. Wiberg, J. Snoonian, M. J. Frisch, J. Phys. Chem. 1998, 102, 16 098, the calculated volume contains 90 % of the total electron density.