Efficient, Copper-Catalyzed, Aerobic Oxidation of Primary Alcohols†
István E. Markó Prof. Dr.
Université catholique de Louvain, Département de Chimie, Bâtiment Lavoisier, Place Louis Pasteur 1, 1348 Louvain-la-Neuve, Belgium, Fax: (+32) 10-47-2788
Search for more papers by this authorArnaud Gautier Dr.
Université catholique de Louvain, Département de Chimie, Bâtiment Lavoisier, Place Louis Pasteur 1, 1348 Louvain-la-Neuve, Belgium, Fax: (+32) 10-47-2788
Search for more papers by this authorRaphaël Dumeunier
Université catholique de Louvain, Département de Chimie, Bâtiment Lavoisier, Place Louis Pasteur 1, 1348 Louvain-la-Neuve, Belgium, Fax: (+32) 10-47-2788
Search for more papers by this authorKanae Doda
Université catholique de Louvain, Département de Chimie, Bâtiment Lavoisier, Place Louis Pasteur 1, 1348 Louvain-la-Neuve, Belgium, Fax: (+32) 10-47-2788
Search for more papers by this authorFreddi Philippart
Université catholique de Louvain, Département de Chimie, Bâtiment Lavoisier, Place Louis Pasteur 1, 1348 Louvain-la-Neuve, Belgium, Fax: (+32) 10-47-2788
Search for more papers by this authorStephen M. Brown Dr.
Zeneca Process Technology Department, Huddersfield Works, P.O. Box A38, Leeds Road, Huddersfield HD2 1FF, UK
Search for more papers by this authorChristopher J. Urch Dr.
Zeneca Agrochemicals, Jealott's Hill Research Station, Bracknell, Berkshire RG42 6ET, UK
Search for more papers by this authorIstván E. Markó Prof. Dr.
Université catholique de Louvain, Département de Chimie, Bâtiment Lavoisier, Place Louis Pasteur 1, 1348 Louvain-la-Neuve, Belgium, Fax: (+32) 10-47-2788
Search for more papers by this authorArnaud Gautier Dr.
Université catholique de Louvain, Département de Chimie, Bâtiment Lavoisier, Place Louis Pasteur 1, 1348 Louvain-la-Neuve, Belgium, Fax: (+32) 10-47-2788
Search for more papers by this authorRaphaël Dumeunier
Université catholique de Louvain, Département de Chimie, Bâtiment Lavoisier, Place Louis Pasteur 1, 1348 Louvain-la-Neuve, Belgium, Fax: (+32) 10-47-2788
Search for more papers by this authorKanae Doda
Université catholique de Louvain, Département de Chimie, Bâtiment Lavoisier, Place Louis Pasteur 1, 1348 Louvain-la-Neuve, Belgium, Fax: (+32) 10-47-2788
Search for more papers by this authorFreddi Philippart
Université catholique de Louvain, Département de Chimie, Bâtiment Lavoisier, Place Louis Pasteur 1, 1348 Louvain-la-Neuve, Belgium, Fax: (+32) 10-47-2788
Search for more papers by this authorStephen M. Brown Dr.
Zeneca Process Technology Department, Huddersfield Works, P.O. Box A38, Leeds Road, Huddersfield HD2 1FF, UK
Search for more papers by this authorChristopher J. Urch Dr.
Zeneca Agrochemicals, Jealott's Hill Research Station, Bracknell, Berkshire RG42 6ET, UK
Search for more papers by this authorFinancial support of this work by the Université catholique de Louvain, the Fonds National de la Recherche Scientifique (R.D.), the Japan Society for the Promotion of Science (K.D.), and Zeneca Ltd. (Zeneca Strategic Fund) is highly appreciated. I.E.M. is grateful to Zeneca for the Zeneca Fellowship and for the 2003 AstraZeneca European Lectureship.
Graphical Abstract
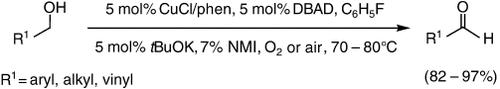
An additive is the key to success: Catalytic amounts of N-methylimidazole are crucial for the aerobic oxidation of primary aliphatic alcohols in the presence of CuCl, 1,10-phenanthroline (phen), and di-tert-butyl azodicarboxylate (DBAD). This reaction, under neutral conditions, yields the aldehydes quantitatively and selectively without overoxidation to the carboxylic acids.
References
- 1
- 1aR. C. Larock in Comprehensive Organic Transformations, VCH, New York, 1989, p. 604;
- 1bG. Procter in Comprehensive Organic Synthesis, Vol. 7 (Eds.: ), Pergamon, Oxford, 1991, p. 305;
10.1016/B978-0-08-052349-1.00192-X Google Scholar
- 1cS. V. Ley, A. Madin in Comprehensive Organic Synthesis, Vol. 7 (Eds.: ), Pergamon, Oxford, 1991, p. 251;
- 1dT. V. Lee in Comprehensive Organic, Synthesis, Vol. 7 (Eds.: ), Pergamon, Oxford, 1991, p. 291.
10.1016/B978-0-08-052349-1.00191-8 Google Scholar
- 2
- 2aR. A. Sheldon, J. K. Kochi in Metal-Catalyzed Oxidations of Organic Compounds, Academic Press, New York, 1981;
- 2bS. V. Ley, J. Norman, W. P. Griffith, S. P. Marsden, Synthesis 1994, 639;
- 2cS.-I. Murahashi, T. Naota, Y. Oda, N. Hirai, Synlett 1995, 733.
- 3
- 3aR. A. Sheldon in Dioxygen Activation and Homogeneous Catalytic Oxidation (Ed.: ), Elsevier, Amsterdam, 1991, p. 573;
- 3bG. Strukul in Catalytic Oxidations with Hydrogen Peroxide as Oxidant, Kluwer Academic Publishers, London, 1992;
- 3cK. Sato, J. Takagi, M. Aoki, R. Noyori, Tetrahedron Lett. 1998, 39, 7549;
- 3dK. Sato, M. Aoki, R. Noyori, Science 1998, 281, 1646;
- 3eA. Berkessel, C. A. Sklorz, Tetrahedron Lett. 1999, 40, 7965.
- 4R. A. Sheldon, Pure Appl. Chem. 2000, 72, 1233, and references therein.
- 5For an excellent review, see: P. T. Anastas, M. M. Kirchhoff, Acc. Chem. Res. 2002, 35, 686, and references therein.
- 6
- 6aK. Masutani, T. Uchida, R. Irie, T. Katsuki, Tetrahedron Lett. 2000, 41, 5119;
- 6bM. Lee, S. Chang, Tetrahedron Lett. 2000, 41, 7507;
- 6cR. A. Sheldon, I. W. C. E. Arends, A. Dijksman, Catal. Today 2000, 57, 157;
- 6dM. Matsumoto, S. Ito, J. Chem. Soc. Chem. Commun. 1981, 907;
- 6eR. Lenz, S. V. Ley, J. Chem. Soc. Perkin Trans. 1. 1997, 3291;
- 6fB. Hinzen, R. Lenz, S. V. Ley, Synthesis 1998, 977;
- 6gA. Bleloch, B. F. G. Johnson, S. V. Ley, A. J. Price, D. S. Shephard, A. W. Thomas, Chem. Commun. 1999, 1907.
- 7
- 7aA. Dijksman, A. Marino-Gonzalez, A. Mairata i Payeras, I. W. C. E. Arends, R. A. Sheldon, J. Am. Chem. Soc. 2001, 123, 6826;
- 7bS.-I. Murahashi, T. Naota, N. Hirai, J. Org. Chem. 1993, 58, 7318;
- 7cT. Matsushita, K. Ebitani, K. Kaneda, Chem. Commun. 1999, 265;
- 7dfor an excellent review, see: R. A. Sheldon, I. W. C. E. Arends, G.-J. ten Brink, A. Dijksman, Acc. Chem. Res. 2002, 35, 774.
- 8
- 8aT. Iwahama, S. Sakaguchi, Y. Nishiyama, Y. Ishii, Tetrahedron Lett. 1995, 36, 6923;
- 8bA. Hanyu, E. Takezawa, S. Sakaguchi, Y. Ishii, Tetrahedron Lett. 1998, 39, 5557;
- 8cT. Iwahama, Y. Yoshino, T. Keitoku, S. Sakaguchi, Y. Ishii, J. Org. Chem. 2000, 65, 6502.
- 9
- 9aT. F. Blackburn, J. Schwarts, Chem. Commun. 1997, 157;
- 9bK. Kaneda, Y. Fujie, K. Ebitani, Tetrahedron Lett. 1997, 38, 9023;
- 9cG.-J. ten Brink, I. W. C. E. Arends, R. A. Sheldon, Science 2000, 287, 1636;
- 9dK. Hallman, C. Moberg, Adv. Synth. Catal. 2001, 343, 260;
- 9eT. Nishimura, T. Onoue, K. Ohe, S. Uemura, J. Org. Chem. 1999, 64, 6750;
- 9fN. Kakiuchi, Y. Maeda, T. Nishimura, S. Uemura, J. Org. Chem. 2001, 66, 6620;
- 9gM. J. Schultz, C. C. Park, M. S. Sigman, Chem. Commun. 2002, 3034;
- 9hK. Yamaguchi, N. Mizuno, Angew. Chem. 2002, 114, 4720;
10.1002/1521-3757(20021202)114:23<4720::AID-ANGE4720>3.0.CO;2-P Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4538.10.1002/1521-3773(20021202)41:23<4538::AID-ANIE4538>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 10See for example, refs. [7a, 7d, 9a, 9e, and 9f].
- 11
- 11aI. E. Markó, P. R. Giles, M. Tsukazaki, S. M. Brown, C. J. Urch, Science 1996, 274, 2044;
- 11bI. E. Markó, P. R. Giles, M. Tsukazaki, I. Chellé-Regnaut, C. J. Urch, S. M. Brown, J. Am. Chem. Soc. 1997, 119, 12 661;
- 11cI. E. Markó, P. R. Giles, M. Tsukazaki, S. M. Brown, C. J. Urch in Transition Metals for Organic Synthesis, Vol. 2 (Eds.: ), 1998, chap. 2. 12, p. 350.
- 12
- 12aI. E. Markó, A. Gautier, I. Chellé-Regnaut, P. R. Giles, M. Tsukazaki, C. J. Urch, S. M. Brown, J. Org. Chem. 1998, 63, 7576;
- 12bI. E. Markó, A. Gautier, J.-L. Mutonkole, R. Dumeunier, A. Ates, C. J. Urch, S. M. Brown, J. Organomet. Chem. 2001, 624, 344.
- 13
- 13aK. D. Karlin, Y. Gultneh, Prog. Inorg. Chem. 1987, 35, 219;
- 13bA. M. Sakharov, I. P. Skibida, Kinet. Catal. 1988, 29, 96;
- 13cN. Kitajima, K. Fujisawa, C. Fujimoto, Y. Moro-oka, S. Hashimoto, T. Kitagawa, K. Toriumi, K. Tatsumi, A. Nakamura, J. Am. Chem. Soc. 1992, 114, 1277;
- 13dE. I. Solomon, U. M. Sundaram, T. E. Machonkin, Chem. Rev. 1996, 96, 2563.
- 14The overoxidation of the aldehyde into the corresponding carboxylic acid has never been observed with this aerobic oxidation protocol. Whilst no proper explanation can be provided at this stage, it is possible that the copper catalyst protects the aldehyde towards further reaction with dioxygen. A similar observation has been reported by Sheldon et al.[7a]
- 15Whilst quantitative conversion of 3 into 4 occurred in the absence and presence of 7 mol % NMI, the oxidation of 3 proceeded more slowly in the presence of this additive (87 % conversion after 30 min in the absence of NMI and 75 % conversion after 30 min in the presence of NMI). The coordination of NMI to copper results in a slower exchange with the excess DBAD and hence, in a longer reaction time.
- 16Studies performed on the anaerobic version of this catalytic system revealed that aliphatic primary alcohols were oxidized with the same efficiency as all the other classes of alcohols, thus ruling out complexes A, B, and E as the culprit for the decomposition pathway. Whilst we could not experimentally rule out complex D, coordination of an alcohol to D should involve the participation of a pentacoordinated copper species. Whilst these are not uncommon, their formation requires a higher activation energy than the coordination to C.
- 17This hydrogen transfer is essentially an intramolecular acid–base reaction. The hydrogen on the coordinated alcohol function is acidified by coordination to the copper centre whilst the hydrazine ligand has basic properties. The elimination of the hydrazine substituent is irreversible under these neutral conditions. Indeed, in the absence of excess base, DBADH2 is unable to displace the alkoxo ligand from the copper complex G.
- 18We have previously demonstrated[11a] that G was not a competent catalyst in the aerobic oxidation protocol when R=alkyl. Under anaerobic conditions, that is, in the presence of 1 equiv DBAD, G can efficiently regenerate the loaded ternary complex A and smooth oxidation ensues.
- 19I. E. Markó, M. Tsukazaki, P. R. Giles, S. M. Brown, C. J. Urch, Angew. Chem. 1997, 109, 2297;
10.1002/ange.19971092011 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 2208.
- 20In full accord with this mechanistic rationale, the use of NMI and other heterocyclic nitrogen derivatives allows the preferential kinetic oxidation of primary aliphatic alcohols over secondary ones. Whilst the selectivities are not yet perfect, initial experiments have shown that the nature of the additive strongly affects the selectivity of this oxidation.