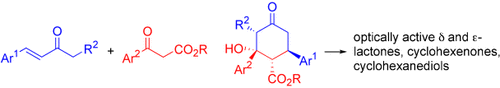
Highly Enantio- and Diastereoselective Organocatalytic Asymmetric Domino Michael–Aldol Reaction of β-Ketoesters and α,β-Unsaturated Ketones†
Nis Halland Dr.
The Danish National Research Foundation: Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax:(+45) 8919-6199
Search for more papers by this authorPompiliu S. Aburel Dr.
The Danish National Research Foundation: Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax:(+45) 8919-6199
Search for more papers by this authorKarl Anker Jørgensen Prof. Dr.
The Danish National Research Foundation: Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax:(+45) 8919-6199
Search for more papers by this authorNis Halland Dr.
The Danish National Research Foundation: Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax:(+45) 8919-6199
Search for more papers by this authorPompiliu S. Aburel Dr.
The Danish National Research Foundation: Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax:(+45) 8919-6199
Search for more papers by this authorKarl Anker Jørgensen Prof. Dr.
The Danish National Research Foundation: Center for Catalysis, Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark, Fax:(+45) 8919-6199
Search for more papers by this authorWe are grateful to Dr. R. G. Hazell for X-ray crystallographic analysis of compounds 6 a, c, e. This work was made possible by a grant from The Danish National Research Foundation.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z53364_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Comprehensive Asymmetric Catalysis (Eds.: ), Springer, Berlin, 1999.
- 2For recent examples of catalytic enantioselective domino reactions in which multiple stereogenic centers are formed, see:
- 2aM. P. Sibi, J. Chen, J. Am. Chem. Soc. 2001, 123, 9472;
- 2bL. A. Arnold, R. Naasz, A. J. Minnaard, B. L. Feringa, J. Org. Chem. 2002, 67, 7244;
- 2cH. M. L. Davies, B. D. Doan, J. Org. Chem. 1999, 64, 8501;
- 2dD. F. Cauble, J. D. Gipson, M. J. Krische, J. Am. Chem. Soc. 2003, 125, 1110;
- 2eJ. Tian, N. Yamagiwa, S. Matsunaga, M. Shibasaki, Angew. Chem. 2002, 114, 3788;
Angew. Chem. Int. Ed. 2002, 41, 3636;
10.1002/1521-3773(20021004)41:19<3636::AID-ANIE3636>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 2fA. Alexakis, S. March, J. Org. Chem. 2002, 67, 8753;
- 2gO. Knopff, A. Alexakis, Org. Lett. 2002, 4, 3835;
- 2hS.-I. Watanabe, A. Córdova, F. Tanaka, C. F. Barbas III, Org. Lett. 2000, 2, 4519;
- 2iA. Córdova, C. F. Barbas III, Tetrahedron Lett. 2003, 44, 1923;
- 2jN. Halland, T. Velgaard, K. A. Jørgensen, J. Org. Chem. 2003, 68, 5067.
- 3For some recent examples of direct catalytic asymmetric cycloaddition reactions, see:
- 3aW. S. Jen, J. J. M. Wiener, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 9874;
- 3bA. B. Northrup, D. W. C. MacMillan, J. Am. Chem. Soc. 2002, 124, 2458;
- 3cK. A. Ahrendt, C. J. Borths, D. W. C. MacMillan, J. Am. Chem. Soc. 2000, 122, 4243;
- 3dK. Juhl, K. A. Jørgensen, Angew. Chem. 2003, 115, 1536;
10.1002/ange.200250652 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1498;
- 3eA. S. Gothelf, K. V. Gothelf, R. G. Hazell, K. A. Jørgensen, Angew. Chem. 2002, 114, 4410;
10.1002/1521-3757(20021115)114:22<4410::AID-ANGE4410>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4236;10.1002/1521-3773(20021115)41:22<4236::AID-ANIE4236>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 3fK. A. Jørgensen, Angew. Chem. 2000, 112, 3702;
10.1002/1521-3757(20001016)112:20<3702::AID-ANGE3702>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3558, and references therein;10.1002/1521-3773(20001016)39:20<3558::AID-ANIE3558>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 3gJ. S. Johnson, D. A. Evans, Acc. Chem. Res. 2000, 33, 325, and references therein;
- 3hK. Gademann, D. E. Chavez, E. N. Jacobsen, Angew. Chem. 2002, 114, 3185;
10.1002/1521-3757(20020816)114:16<3185::AID-ANGE3185>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3059;10.1002/1521-3773(20020816)41:16<3059::AID-ANIE3059>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 3iE. J. Corey, Angew. Chem. 2002, 114, 1724;
10.1002/1521-3757(20020517)114:10<1724::AID-ANGE1724>3.0.CO;2-Q Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1650;10.1002/1521-3773(20020517)41:10<1650::AID-ANIE1650>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 3jD. A. Evans, J. Wu, J. Am. Chem. Soc. 2003, 125, 10 162;
- 3kD. B. Ramachary, N. S. Chowdari, C. F. Barbas III, Angew. Chem. 2003, 115, 4365;
10.1002/ange.200351916 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4233;
- 3l Cycloaddition Reactions in Organic Synthesis (Eds.: ), Wiley-VCH, 2000.
- 4N. Halland, P. S. Aburel, K. A. Jørgensen, Angew. Chem. 2003, 115, 685;
10.1002/ange.200390150 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 661.
- 5N. Halland, T. Hansen, K. A. Jørgensen, Angew. Chem. 2003, 115, 4955; Angew. Chem. Int. Ed. 2003, 42, 5105.
- 6N. Halland, R. G. Hazell, K. A. Jørgensen, J. Org. Chem. 2002, 67, 8331.
- 7
- 7aU. Eder, G. Sauer, R. Wiechert, Angew. Chem. 1971, 83, 492;
10.1002/ange.19710831307 Google ScholarAngew. Chem. Int. Ed. Engl. 1971, 10, 496;
- 7bZ. G. Hajos, D. R. Parrish, J. Org. Chem. 1974, 39, 1615; see also;
- 7cT. Arai, H. Sasai, K.-I. Aoe, K. Okamura, T. Date, M. Shibasaki, Angew. Chem. 1996, 108, 103;
10.1002/ange.19961080123 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 104;
- 7dT. Bui, C. F. Barbas III, Tetrahedron Lett. 2000, 41, 6951.
- 8
- 8aW. Dieckmann, K. Fischer, Ber. Dtsch. Chem. Ges. 1911, 44, 966;
- 8bJ. Christoffers, J. Chem. Soc. Perkin Trans. 1, 1997, 3141, see also: F. Tanaka, R. Thayumanavan, C. F. Barbas III, J. Am. Chem. Soc. 2002, 124, 8523.
- 9The 4,5-diphenyl-imidazolidine-2-carboxylic acid catalyst recently reported for the catalytic enantioselective formation of warfarin,[5] also promoted the domino Michael–aldol reaction in up to 90 % ee in moderate yield.
- 10CCDC-224 447 (6 a), CCDC-224 448 (6 c), and CCDC-224 449 (6 e) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 11Baeyer-Villiger oxidations generally occur with retention of stereochemistry at the migrating center; see, for example: Comprehensive Organic Synthesis (Ed.: ), Pergamon, Berlin, 1991.
- 12Inversion of the α-carbonyl stereogenic center was observed during the translactonization procedure.
- 13M. S. Cooper, H. Heaney, A. J. Newbold, W. R. Sanderson, Synlett 1990, 533.
- 14The reaction rate is strongly concentration-dependent (see Supporting Information).