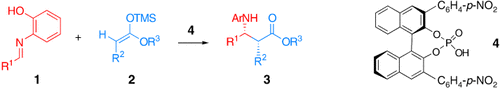
Enantioselective Mannich-Type Reaction Catalyzed by a Chiral Brønsted Acid†
This work was supported by a Grant-in-Aid for Scientific Research (No. 15550042) from the Ministry of Education, Science, Sports, Culture, and Technology, Japan.
Graphical Abstract
The enantioselective Mannich-type reaction of an enolate or an enolate anion equivalent with aldimines constitutes a useful method for the preparation of chiral β-amino carbonyl compounds, which are the precursors of biologically important compounds such as β-lactams and β-amino acids. The development of chiral catalysts for the asymmetric Mannich-type reaction has attracted the attention of synthetic organic chemists.1 Although stoichiometric amounts of chiral acid were employed initially,2 a number of enantioselective catalysts such as chiral Lewis acid catalysts3 and chiral base catalysts4 have been developed lately.
In addition to metal-based chiral catalysts,5 the use of small organic molecules as catalysts to promote asymmetric reactions has emerged as a new frontier in reaction methodology.6 Accordingly, L-proline derivatives7 and peptide derivatives8 have been developed as catalysts for the Mannich-type reactions. We previously reported that Mannich-type reactions9 and the aza-Diels–Alder reaction10 proceed smoothly in the presence of a catalytic amount of a strong Brønsted acid. We thus postulated that the use of a chiral Brønsted acid, in which the proton is surrounded by bulky substituents, may lead to effective asymmetric induction. We report herein an enantioselective Mannich-type reaction of silyl enolates with aldimines catalyzed by a chiral metal-free Brønsted acid.11, 12
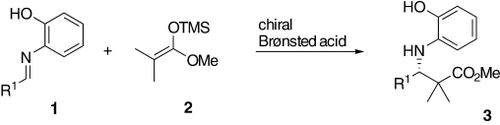
First, treatment of aldimine 1 a (Scheme 1, R1=Ph) and ketene silyl acetal 2 (3.0 equiv) with 0.3 equivalents of the chiral phosphate 4 a13, 14, 15 (which is readily prepared from (R)-BINOL; Scheme 2) in toluene at −78 °C led to a smooth Mannich-type reaction to give 3 a (R1=Ph). However, no enantioselectivity was observed (Table 1, entry 1), as determined by HPLC analysis with a chiral stationary phase column.16 Next, we synthesized chiral phosphates 4 b–e by Suzuki coupling of bis(boronic acid) 517 followed by demethylation and subsequent phosphorylation, as shown in Scheme 2. Introduction of aromatic groups at the 3,3′-positions exerted a beneficial effect on the enantioselectivity. Use of 4 b as a chiral Brønsted acid in toluene increased the enantiofacial selectivity to 27 % ee (Table 1, entry 2). The introduction of 4-nitrophenyl groups (i.e. 4 e) had a dual effect: 1) improvement of enantioselectivity to 87 % ee, 2) acceleration of reaction rate, thereby allowing the reaction to go to completion in 4 h (Table 1, entry 5). The absolute stereochemistry of 3 a was determined by chiral HPLC analysis by comparison of the retention time with that found in the literature.3c
Mannich-type reaction of aldimines 1 and ketene silyl acetals 2 to form β-aminoesters 3.
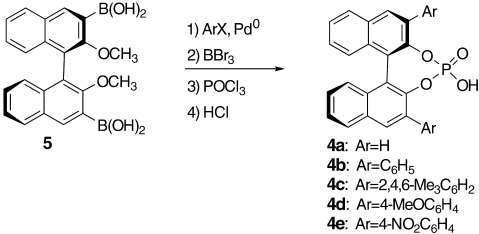
Formation of chiral phosphates 4, readily available from (R)-BINOL.
|
Entry |
Ar |
t [h] |
Yield [%] |
ee [%] |
|---|---|---|---|---|
|
1 |
H |
22 |
57 |
0 |
|
2 |
Ph |
20 |
100 |
27 |
|
3 |
2,4,6-Me3C6H2 |
27 |
100 |
60 |
|
4 |
4-MeOC6H4 |
46 |
99 |
52 |
|
5 |
4-NO2C6H4 |
4 |
96 |
87 |
- [a] Aldimine 1 a (R1=Ph) (1.0 equiv) and 2 (3.0 equiv) were treated with Brønsted acid 4 (30 mol %) in toluene at −78 °C.
By further optimization of the reaction conditions, we found that the use of aromatic solvents led to high enantioselectivities, whereas protic solvents gave racemates.18 Furthermore, a lower loading (10 mol %) of the Brønsted acid was sufficient to retain the high enantioselectivity. The results of the Mannich-type reaction of 2 with several aldimines catalyzed by chiral Brønsted acid 4 e are shown in Table 2. Aldimines derived from aromatic aldehydes afforded adducts with good to high enantioselectivities. The chemical yields were excellent in all cases.19, 20
|
Entry |
R1 |
Product |
Yield [%] |
ee [%] |
|---|---|---|---|---|
|
1 |
Ph |
3 a |
98 |
89 |
|
2 |
p-MeC6H4 |
3 b |
100 |
89 |
|
3 |
p-FC6H4 |
3 c |
100 |
85 |
|
4 |
p-ClC6H4 |
3 d |
100 |
80 |
- [a] Aldimine 1 (1.0 equiv) and 2 (1.5 equiv) were treated with 4 e (10 mol %) in toluene at −78 °C for 24 h.
Next, other ketene silyl acetals were examined (Table 3). Monosubstituted ketene silyl acetals led to high syn selectivity as well as excellent enantioselectivity. The ketene silyl acetal derived from ethyl propionate furnished the corresponding ester in 96 % ee (Table 3, entry 1). Substituted aromatic, heteroaromatic, and α,β-unsaturated aldimines also gave the corresponding adducts with high enantioselectivities (Table 3, entries 2–7). The reactions of ketene silyl acetals derived from ethyl 3-phenylpropionate (Table 3, entries 8–10) and methyl 2-triphenylsilyloxyacetate (Table 3, entry 11) also exhibited excellent syn selectivities21 and high enantioselectivities.
- [a] Aldimine 1 (1.0 equiv) and ketene silyl acetal 6 (1.5 equiv) were treated with 4 e (10 mol %) in toluene at −78 °C for 24 h. [b] ee value of syn isomer. [c] E/Z=87:13. [d] E/Z=87:13. [e] E/Z=91:9.
Because the use of N-benzylideneaniline in place of 1 a (R1=Ph) in the reaction with 2 lowered the enantioselectivity to 39 % ee, we concluded that the presence of the hydroxy group in the ortho position of the aldimine is essential for the present enantioselective Mannich-type reaction. This reaction can be considered to proceed via an iminium salt, generated from the aldimine and the Brønsted acid. Although the precise mechanism has not been elucidated, it is supposed that 3,3′-diaryl groups, which are not coplanar with the naphthyl groups (see 8-1
In summary, we have developed a chiral Brønsted acid catalyzed enantioselective Mannich-type reaction of aldimines with silyl enolates, and β-aminoesters were obtained with high to excellent enantioselectivities under metal-free conditions. This method adds a new entry to the catalogue organo-catalyzed asymmetric reactions. This method can potentially be extended to a variety of enantioselective nucleophilic addition reactions to carbon–nitrogen double bonds. Further investigations to clarify the reaction mechanism and its application to other enantioselective reactions are in progress.
Experimental Section
General procedure (Table 3, Entry 1): A solution of 6 (R2=Me, R3=Et) (50 μL, 0.246 mmol) was added dropwise over 3 min to a solution of 1 (R1=Ph) (32.0 mg, 0.162 mmol) and 4 e (9.5 mg, 0.0161 mmol) in toluene (1 mL) at −78 °C. The reaction was stirred at this temperature for 17 h. The mixture was quenched by the addition of saturated solutions of NaHCO3 and KF at −78 °C. After filtration over celite, the filtrate was extracted with ethyl acetate. The combined organic layers were washed successively washed with HCl (1 n) and brine, dried over anhydrous Na2SO4, and concentrated to dryness. The remaining solid was purified by TLC (SiO2, hexane/EtOAc 3:1) to give 7 (45.6 mg, 0.155 mmol) in 100 % yield. The enantiomeric excess was determined on a Daicel Chiralpak AS-H column.