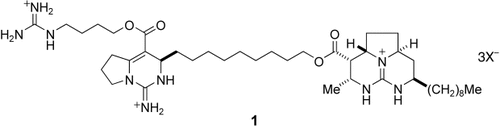
Enantioselective Total Synthesis of Batzelladine A†
Jun Shimokawa
Institute of Molecular and Cellular Biosciences, University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan, Fax: (+81) 3-5841-8495
Search for more papers by this authorKoji Shirai
Institute of Molecular and Cellular Biosciences, University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan, Fax: (+81) 3-5841-8495
Search for more papers by this authorAya Tanatani Dr.
Institute of Molecular and Cellular Biosciences, University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan, Fax: (+81) 3-5841-8495
Search for more papers by this authorYuichi Hashimoto Prof.
Institute of Molecular and Cellular Biosciences, University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan, Fax: (+81) 3-5841-8495
Search for more papers by this authorKazuo Nagasawa Prof.
Institute of Molecular and Cellular Biosciences, University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan, Fax: (+81) 3-5841-8495
Search for more papers by this authorJun Shimokawa
Institute of Molecular and Cellular Biosciences, University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan, Fax: (+81) 3-5841-8495
Search for more papers by this authorKoji Shirai
Institute of Molecular and Cellular Biosciences, University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan, Fax: (+81) 3-5841-8495
Search for more papers by this authorAya Tanatani Dr.
Institute of Molecular and Cellular Biosciences, University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan, Fax: (+81) 3-5841-8495
Search for more papers by this authorYuichi Hashimoto Prof.
Institute of Molecular and Cellular Biosciences, University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan, Fax: (+81) 3-5841-8495
Search for more papers by this authorKazuo Nagasawa Prof.
Institute of Molecular and Cellular Biosciences, University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan, Fax: (+81) 3-5841-8495
Search for more papers by this authorWe thank Prof. T. Nakata (Tokyo University of Science) and Dr. H. Koshino (RIKEN) for helpful discussions. This work has been supported by grants from the Pharmacy Research Encouragement Foundation, the Uehara Memorial Foundation, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Graphical Abstract
One-step formation of an enone from a primary alcohol has been effectively applied in the total synthesis of batzelladine A (1). Successive 1,3-dipolar cycloadditions with a subsequent cyclization were used to form the tricyclic guanidine subunit of this natural product, which has the strongest inhibitory activity of HIV gp120-CD4 binding among the batzelladines.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z53200_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. D. Patil, N. V. Kumar, W. C. Kokke, M. F. Bean, A. J. Freyer, C. De Brosse, S. Mai, A. Truneh, D. J. Faulkner, B. Carté, A. L. Breen, R. P. Hertzberg, R. K. Johnson, J. W. Westley, B. C. M. Potts, J. Org. Chem. 1995, 60, 1182;
- 1bA. D. Patil, A. J. Freyer, P. B. Taylor, B. Carté, G. Zuber, R. K. Johnson, D. J. Faulkner, J. Org. Chem. 1997, 62, 1814.
- 2The identity of the source of these compounds was revised:
- 2aJ. C. Braekman, D. Daloze, R. Tavares, E. Hadju, R. W. M. Van Soest, J. Nat. Prod. 2000, 63, 193;
- 2bR. W. M. Van Soest, J. C. Braekman, D. J. Faulkner, E. Hadju, M. K. Harper, J. Vacelet, Bull. Inst. R. Sci. Nat. Belg. 1996, 66, 89.
- 3A. V. Rama Rao, M. K. Gurjar, J. Vasudevan, J. Chem. Soc. Chem. Commun. 1995, 1369.
- 4S. Louwrier, M. Ostendorf, A. Tuynman, H. Hiemstra, Tetrahedron Lett. 1996, 37, 905.
- 5aB. B. Snider, J. Chen, A. D. Patil, A. J. Freyer, Tetrahedron Lett. 1996, 37, 6977;
- 5bB. B. Snider, J. Chen, Tetrahedron Lett. 1998, 39, 5697;
- 5cB. B. Snider, M. V. Busuyek, J. Nat. Prod. 1999, 62, 1707.
- 6aG. P. Black, P. J. Murphy, N. D. A. Walshe, Tetrahedron 1998, 54, 9481;
- 6bG. P. Black, P. J. Murphy, A. Thornhill, N. D. A. Walshe, C. Zanetti, Tetrahedron 1999, 55, 6547.
- 7
- 7aF. Cohen, L. E. Overman, S. K. L. Sakata, Org. Lett. 1999, 1, 2169;
- 7bF. Cohen, L. E. Overman, J. Am. Chem. Soc. 2001, 123, 10 782;
- 7cA. S. Franklin, S. K. Ly, G. H. Mackin, L. E. Overman, A. J. Shaka, J. Org. Chem. 1999, 64, 1512;
- 7dA. I. McDonald, L. E. Overman, J. Org. Chem. 1999, 64, 1520;
- 7eF. Cohen, S. K. Collins, L. E. Overman, Org. Lett. 2003, 5, 4485.
- 8S. G. Duron, D. Y. Gin, Org. Lett. 2001, 3, 1551.
- 9P. A. Evans, T. Manangan, Tetrahedron Lett. 2001, 42, 6637.
- 10M. C. Elliot, M. S. Long, Tetrahedron Lett. 2002, 43, 9191.
- 11
- 11aK. Nagasawa, H. Koshino, T. Nakata, Tetrahedron Lett. 2001, 42, 4155;
- 11bK. Nagasawa, T. Ishiwata, Y. Hashimoto, T. Nakata, Tetrahedron Lett. 2002, 43, 6383;
- 11cT. Ishiwata, T. Hino, H. Koshino, Y. Hashimoto, T. Nakata, K. Nagasawa, Org. Lett. 2002, 4, 2921.
- 12A. Goti, M. Cacciarini, F. Cardona, A. Brandi, Tetrahedron Lett. 1999, 40, 2853.
- 13
- 13aK. S. Kim, L. Qian, Tetrahedron Lett. 1993, 34, 7677;
- 13bE. J. Iwanowicz, M. A. Poss, J. Lin, Synth. Commun. 1993, 23, 1443.
- 14D. S. Dodd, A. P. Kozikowski, Tetrahedron Lett. 1994, 35, 977.
- 15K. B. Sharpless, R. F. Lauer, A. Y. Teranishi, J. Am. Chem. Soc. 1973, 95, 6137.
- 16
- 16aY. Ito, T. Hirao, T. Saegusa, J. Org. Chem. 1978, 43, 1011;
- 16bJ. Tsuji, I. Minani, I. Shimizu, Tetrahedron Lett. 1983, 24, 5635;
- 16cI. Minani, K. Takahashi, I. Shimizu, T. Kimura, J. Tsuji, Tetrahedron 1986, 42, 2971;
- 16dJ. Tsuji, K. Takahashi, I. Minani, I. Shimizu, Tetrahedron Lett. 1984, 25, 4783.
- 17
- 17aG. A. Kraus, M. J. Taschner, J. Org. Chem. 1980, 45, 1175;
- 17bG. A. Kraus, B. Roth, J. Org. Chem. 1980, 45, 4825;
- 17cB. O. Lindgren, T. Nilsson, Acta Chem. Scand. 1973, 27, 888.
- 18N. Hashimoto, T. Aoyama, T. Shioiri, Chem. Pharm. Bull. 1981, 29, 1475.
- 19K. C. Nicolaou, Y.-L. Zhong, P. S. Baran, J. Am. Chem. Soc. 2000, 122, 7596.
- 20We currently believe the mechanism of this reaction to involve iminium formation by oxidation of the guanidine nitrogen atom at the ring junction, followed by isomerization to form the α,β-unsaturated aldehyde.
- 21Spectral data for synthetic 1: [α]
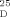 =+4.29 (c=0.25, MeOH) (lit:[1a] [α]
=+4.29 (c=0.25, MeOH) (lit:[1a] [α] =+8.9 (c=2.3, MeOH)); IR (neat):
=+8.9 (c=2.3, MeOH)); IR (neat):  =2925, 2854, 1732, 1697, 1683, 1648, 1637, 1558, 1347, 1092 cm−1; 1H NMR (500 MHz, CD3OD): δ=4.39 (t, J=6.1 Hz, 1 H), 4.21 (t, J=6.4 Hz, 2 H), 4.13 (t, J=6.7 Hz, 2 H), 3.93 (m, 1 H), 3.83 (m, 2 H), 3.66 (m, 1 H), 3.52 (m, 1 H), 3.32 (m, 1 H), 3.22 (t, J=7.3 Hz, 2 H), 3.12 (dd, J=4.6, 3.5 Hz, 1 H), 2.98 (m, 1 H), 2.35 (m, 1 H), 2.28–2.17 (m, 3 H), 2.10 (m, 1 H), 1.76 (m, 2 H), 1.72–1.52 (m, 9 H), 1.48–1.23 (m, 29 H), 1.27 (t, J=6.7 Hz, 3 H), 0.89 ppm (t, J=6.7 Hz, 3 H); 13C NMR (125 MHz, CD3OD): δ=170.7, 166.2, 158.7, 153.1, 152.7, 151.5, 103.3, 66.0, 65.1, 57.7, 57.3, 53.2, 51.2, 49.9, 48.8, 45.6, 42.0, 37.5, 36.9, 34.2, 33.0, 31.9, 31.4, 29.7, 29.3, 27.0, 26.6, 26.2, 25.2, 23.7, 22.9, 18.4, 14.4 ppm; HRMS (FAB, MH+): calcd for C42H74N9O4: 768.5864, found: 768.5866.
=2925, 2854, 1732, 1697, 1683, 1648, 1637, 1558, 1347, 1092 cm−1; 1H NMR (500 MHz, CD3OD): δ=4.39 (t, J=6.1 Hz, 1 H), 4.21 (t, J=6.4 Hz, 2 H), 4.13 (t, J=6.7 Hz, 2 H), 3.93 (m, 1 H), 3.83 (m, 2 H), 3.66 (m, 1 H), 3.52 (m, 1 H), 3.32 (m, 1 H), 3.22 (t, J=7.3 Hz, 2 H), 3.12 (dd, J=4.6, 3.5 Hz, 1 H), 2.98 (m, 1 H), 2.35 (m, 1 H), 2.28–2.17 (m, 3 H), 2.10 (m, 1 H), 1.76 (m, 2 H), 1.72–1.52 (m, 9 H), 1.48–1.23 (m, 29 H), 1.27 (t, J=6.7 Hz, 3 H), 0.89 ppm (t, J=6.7 Hz, 3 H); 13C NMR (125 MHz, CD3OD): δ=170.7, 166.2, 158.7, 153.1, 152.7, 151.5, 103.3, 66.0, 65.1, 57.7, 57.3, 53.2, 51.2, 49.9, 48.8, 45.6, 42.0, 37.5, 36.9, 34.2, 33.0, 31.9, 31.4, 29.7, 29.3, 27.0, 26.6, 26.2, 25.2, 23.7, 22.9, 18.4, 14.4 ppm; HRMS (FAB, MH+): calcd for C42H74N9O4: 768.5864, found: 768.5866.