Redox-Active Organometallic Vesicles: Aqueous Self-Assembly of a Diblock Copolymer with a Hydrophilic Polyferrocenylsilane Polyelectrolyte Block†
K. Nicole Power-Billard
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6, Canada, Fax: (+1) 416-978-6157
Search for more papers by this authorRichard J. Spontak Prof.
Departments of Chemical Engineering and Materials Science & Engineering, North Carolina State University, Raleigh, NC 27695, USA, Fax: (+1) 919-515-3465
Search for more papers by this authorIan Manners Prof.
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6, Canada, Fax: (+1) 416-978-6157
Search for more papers by this authorK. Nicole Power-Billard
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6, Canada, Fax: (+1) 416-978-6157
Search for more papers by this authorRichard J. Spontak Prof.
Departments of Chemical Engineering and Materials Science & Engineering, North Carolina State University, Raleigh, NC 27695, USA, Fax: (+1) 919-515-3465
Search for more papers by this authorIan Manners Prof.
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario, M5S 3H6, Canada, Fax: (+1) 416-978-6157
Search for more papers by this authorI.M. thanks the Canadian Government for a Canada Research Chair and would like to thank the Natural Sciences and Engineering Research Council (NSERC) for support. R.J.S. thanks the STC Program of the National Science Foundation under Agreement No. CHE-9876674 for support. Special thanks to Mr. Fred Pearson at McMaster University for the TEM analysis of LN2-quenched solutions of polymer 4. We thank Alex Bartole-Scott and John Halfyard for performing the electrochemistry experiments.
Graphical Abstract
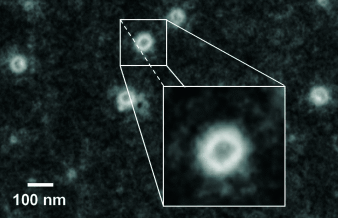
A water-soluble poly(dimethylsiloxane-b-ferrocenylsilane) diblock copolymer containing a hydrophilic cationic polyferrocene block, which is formed in a self-assembly process, yields redox-active vesicles with a diameter of approximately 85 nm (see figure) in which the organometallic block is located on both the outside and the inside of the formed aggregates.
References
- 1
- 1aM. Antonietti, C. Göltner, Angew. Chem. 1997, 109, 944; Angew. Chem. Int. Ed. Engl. 1997, 36, 910;
- 1bP. Alexandridis, R. J. Spontak, Curr. Opin. Colloid Interface Sci. 1999, 4, 130;
- 1cP. Alexandridis, B. Lindman, Amphiphilic Block Copolymers: Self-Assembly and Applications, Elsevier, Amsterdam, 2000.
- 1dS. Förster, B. Berton, H. P. Hentze, E. Krämer, M. Antonietti, P. Lindner, Macromolecules 2001, 34, 4610.
- 2For examples of recent work on nonspherical morphologies of block copolymers in solution, see
- 2aL. Zhang, A. Eisenberg, J. Am. Chem. Soc. 1996, 118, 3168;
- 2bK. Yu, C. Bartels, A. Eisenberg, Langmuir 1999, 15, 7157;
- 2cX. H. Yan, G. J. Liu, F. T. Liu, B. Z. Tang, H. Peng, A. B. Pakhomov, C. Y. Wong, Angew. Chem. 2001, 113, 3705;
Angew. Chem. Int. Ed. 2001, 40, 3593;
10.1002/1521-3773(20011001)40:19<3593::AID-ANIE3593>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 2dY. Y. Won, H. D. Davis, F. S. Bates, Science 1999, 283, 960;
- 2eS. Stewart, G. Liu, Angew. Chem. 2000, 112, 348;
Angew. Chem. Int. Ed. 2000, 39, 340;
10.1002/(SICI)1521-3773(20000117)39:2<340::AID-ANIE340>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 2fL. Zhang, A. Eisenberg, Science 1995, 268, 1728;
- 2gJ. P. Spatz, S. Mössmer, M. Möller, Angew. Chem. 1996, 108, 1673;
10.1002/ange.19961081343 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1510.
- 3
- 3aA.-Y. A. I. Lebdeh, P. M. Budd, V. M. Nace, J. Mater. Chem. 1998, 8, 1839;
- 3bR. Gref, Y. Minamitake, M. T. Peracchia, V. Trubetskoy, V. Torchilin, R. Langer, Science 1994, 263, 1600.
- 4
- 4aS. Y. Liu, S. P. Armes, Angew. Chem. 2002, 114, 1471;
Angew. Chem. Int. Ed. 2002, 41, 1413.
10.1002/1521-3773(20020415)41:8<1413::AID-ANIE1413>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 4bS. Y. Liu, N. C. Billingham, S. P. Armes, Angew. Chem. 2001, 113, 2390;
Angew. Chem. Int. Ed. 2001, 40, 2328;
10.1002/1521-3773(20010618)40:12<2328::AID-ANIE2328>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 4cV. Bütün, S. P. Armes, N. C. Billingham, Z. Tuzar, A. Rankin, J. Eastoe, R. K. Heenan, J. Am. Chem. Soc. 2001, 123, 9910;
- 4dA. V. Kabanov, T. K. Bornich, V. A. Kabanov, K. Yu, A. Eisenberg, J. Am. Chem. Soc. 1998, 120, 9941;
- 4eJ. Zhu, A. Eisenberg, R. B. Lennox, J. Am. Chem. Soc. 1991, 113, 5583;
- 4fF. Henselwood, G. Liu, Macromolecules 1997, 30, 488;
- 4gJ. F. Gohy, N. Willet, S. Varshney, J. X. Zhang, R. Jerome, Angew. Chem. 2001, 113, 3314;
Angew. Chem. Int. Ed. 2001, 40, 3214;
10.1002/1521-3773(20010903)40:17<3214::AID-ANIE3214>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 4hK. L. Wooley, Chem. Eur. J. 1997, 3, 1397.
- 5Recent works on the self-assembly of metal-containing block copolymers include
- 5aS. J. Hou, W. K. Chan, Macromol. Rapid Commun. 1999, 20, 440;
10.1002/(SICI)1521-3927(19990801)20:8<440::AID-MARC440>3.0.CO;2-R CAS Web of Science® Google Scholar
- 5bS. J. Hou, K. Y. K. Man, W. K. Chan, Langmuir 2003, 19, 2485;
- 5cC. M. Park, J. E. McAlvin, C. L. Fraser, E. L. Thomas, Chem. Mater. 2002, 14, 1225;
- 5dJ. F. Gohy, B. G. G. Lohmeijer, S. K. Varshney, U. S. Schubert, Macromolecules 2002, 35, 7427;
- 5eJ. F. Gohy, H. Hofmeier, A. Alexeev, U. S. Schubert, Macromol. Chem. Phys. 2003, 204, 1524.
- 6I. Manners, Science 2001, 294, 1664.
- 7
- 7aR. Rulkens, Y. Z. Ni, I. Manners, J. Am. Chem. Soc. 1994, 116, 12 121;
- 7bY. Ni, R. Rulkens, I. Manners, J. Am. Chem. Soc. 1996, 118, 4102.
- 8
- 8aJ. A. Massey, K. N. Power, M. A. Winnik, I. Manners, Adv. Mater. 1998, 10, 1559;
10.1002/(SICI)1521-4095(199812)10:18<1559::AID-ADMA1559>3.0.CO;2-J CAS Web of Science® Google Scholar
- 8bJ. A. Massey, M. A. Winnik, I. Manners, V. Z.-H. Chan, J. M. Ostermann, R. Enchelmaier, J. P. Spatz, M. Möller, J. Am. Chem. Soc. 2001, 123, 3147;
- 8cJ. Raez, I. Manners, M. A. Winnik, J. Am. Chem. Soc. 2002, 124, 10 381.
- 9
- 9aL. Luo, A. Eisenberg, J. Am. Chem. Soc. 2001, 123, 1012;
- 9bD. E. Discher, A. Eisenberg, Science 2002, 297, 967.
- 10
- 10aP. Gómez-Elipe, P. M. Macdonald, I. Manners, Angew. Chem. 1997, 109, 780;
10.1002/ange.19971090721 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 762;
- 10bP. Gómez-Elipe, R. Resendes, P. M. Macdonald, I. Manners, J. Am. Chem. Soc. 1998, 120, 8348.
- 11K. N. Power-Billard, I. Manners, Macromol. Rapid Commun. 2002, 23, 607.
- 12P. Echlin, Low-Temperature Microscopy and Analysis, Plenum, New York, 1992.
10.1007/978-1-4899-2302-8 Google Scholar
- 13P. Terech, A. de Geyer, B. Struth, Y. Talmon, Adv. Mater. 2002, 14, 495.
- 14J. R. Harris, Negative Staining and Cryoelectron Microscopy: The Thin Film Techniques, Bios Scientific, Oxford, 1997.
10.1017/S1551929500060016 Google Scholar
- 15
- 15aA. Du Chesne, Macromol. Chem. Phys. 1999, 200, 1813;
10.1002/(SICI)1521-3935(19990801)200:8<1813::AID-MACP1813>3.0.CO;2-N CAS Web of Science® Google Scholar
- 15bR. Thomann, R. J. Spontak in Science, Technology and Education of Microscopy: An Overview (Ed.: ), Formatex, Badajoz, Spain, 2003, pp. 249–254.
- 16
- 16aY. Kakizawa, H. Sakai, A. Yamaguchi, Y. Kondo, N. Yoshino, M. Abe, Langmuir 2001, 17, 8044;
- 16bT. Saji, K. Hoshino, S. Aoyagui, J. Am. Chem. Soc. 1985, 107, 6865.
- 17The presence of a single wave is anticipated based on the formation of ion pairs between ferrocenium units and the chloride anions in the supporting electrolyte, which reduces any metal–metal interactions present: see
- 17aR. Rulkens, A. J. Lough, I. Manners, S. R. Lovelace, C. Grant, W. E. Geiger, J. Am. Chem. Soc. 1996, 118, 12 683;
- 17bF. Barriere, N. Camire, W. E. Geiger, U. T. Mueller-Westerhoff, R. Sanders, J. Am. Chem. Soc. 2002, 124, 7262.
- 18K. N. Power-Billard, T. J. Peckham, A. Butt, F. Jäkle, I. Manners, J. Inorg. Organomet. Polym. 2000, 10, 157.
- 19Halide substitution reactions on PFS materials have previously been shown to take place without any significant molecular-weight decline. See, for example,
- 19aK. N. Power-Billard, I. Manners, Macromolecules 2000, 33, 26;
- 19bD. L. Zechel, K. C. Hultszch, R. Rulkens, B. Balaishis, Y. Ni, J. K. Pudelski, A. J. Lough, I. Manners, Organometallics 1996, 15, 1972.
- 20J. A. Massey, K. Kulbaba, M. A. Winnik, I. Manners, J. Polym. Sci. Polym. Phys. Part B 2000, 28, 3032.