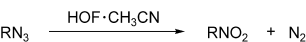Direct Oxidation of Azides to Nitro Compounds
G. K. Surya Prakash Prof. Dr.
Loker Hydrocarbon Research Institute and Department of Chemistry, University of Southern California, 837 Bloom Walk, Los Angeles, CA 90034-1661, USA, Fax: (+1) 213-740-6270
Search for more papers by this authorMarkus Etzkorn Dr.
Loker Hydrocarbon Research Institute and Department of Chemistry, University of Southern California, 837 Bloom Walk, Los Angeles, CA 90034-1661, USA, Fax: (+1) 213-740-6270
Search for more papers by this authorG. K. Surya Prakash Prof. Dr.
Loker Hydrocarbon Research Institute and Department of Chemistry, University of Southern California, 837 Bloom Walk, Los Angeles, CA 90034-1661, USA, Fax: (+1) 213-740-6270
Search for more papers by this authorMarkus Etzkorn Dr.
Loker Hydrocarbon Research Institute and Department of Chemistry, University of Southern California, 837 Bloom Walk, Los Angeles, CA 90034-1661, USA, Fax: (+1) 213-740-6270
Search for more papers by this authorGraphical Abstract
References
- 1
- 1a Houben-Weyl: Methoden der Organischen Chemie, Vol. 10/1 (Ed.: ), Thieme, Stuttgart, 1971; Houben-Weyl: Methoden der Organischen Chemie, Vol. E16D/1 (Ed.: ), Thieme, Stuttgart, 1992;
- 1bThe Chemistry of the Nitro and Nitroso Group (Ed.: H. Feuer), Wiley Interscience, New York, 1969, Part 1, 1970 Part 2, and 1982 Supplement F.
- 2
- 2aN. Ono, The Nitro Group in Organic Synthesis, Wiley-VCH, New York, 2001;
10.1002/0471224480 Google Scholar
- 2bH. Feuer, A. T. Nielson, Nitro Compounds: Recent Advances in Synthesis and Chemistry, VCH, New York, 1990;
- 2cK. B. G. Torssell, Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis: Novel Strategies in Synthesis, VCH, New York, 1988.
- 3
- 3aG. A. Olah, R. Malhotra, S. C. Narang, Nitration: Methods and Mechanisms, VCH, New York, 1989;
- 3bK. Schofield, Aromatic Nitrations, Cambridge University Press, Cambridge, 1980.
- 4For individual conditions and reagents, see references [1–3] and references therein.
- 5E. J. Corey, B. Samuelsson, F. A. Luzzio, J. Am. Chem. Soc. 1984, 106, 3682–3683.
- 6S. Rozen, M. Carmeli, J. Am. Chem. Soc. 2003, 125, 8118–8119.
- 7
- 7aS. Dayan, I. Ben-David, S. Rozen, J. Org. Chem. 2000, 65, 8816–8818;
- 7bS. Rozen, Y. Bareket, S. Dayan, Tetrahedron Lett. 1996, 37, 531–534.
- 8S. Rozen, S. Dayan, Y. Bareket, J. Org. Chem. 1995, 60, 8267–8269.
- 9S. Rozen, Y. Bareket, M. Kol, Tetrahedron 1993, 49, 8169–8178.
- 10
- 10aS. Rozen, Y. Bareket, J. Org. Chem. 1997, 62, 1457–1462;
- 10bA. Toyota, Y. Ono, J. Chiba, T. Sugihara, C. Kaneko, Chem. Pharm. Bull. 1996, 44, 703–708.
- 11
- 11aS. M. Dirk, E. T. Mickelson, J. C. Henderson, J. M. Tour, Org. Lett. 2000, 2, 3405–3406;
- 11bS. Rozen, M. Kol, J. Org. Chem. 1992, 57, 7342–7344;
- 11cM. Kol, S. Rozen, J. Chem. Soc. Chem. Commun. 1991, 567–568.
- 12S. Rozen, A. Bar-Haim, E. Mishami, J. Org. Chem. 1994, 59, 1208–1209.
- 13
- 13aS. Dayan, M. Kol, S. Rozen, Synthesis 1999, 1427–1430;
- 13bD. E. Chavez, M. A. Hiskey, J. Energ. Mater. 1999, 17, 357–377.
- 14
- 14aS. Rozen, Acc. Chem. Res. 1996, 29, 243–248;
- 14bS. Rozen, Pure Appl. Chem. 1999, 71, 481–487.
- 15E. H. Appelman, R. C. Thomson, A. G. Engelkemeir, Inorg. Chem. 1979, 18, 909–911.
- 16
- 16aE. H. Appelman, Acc. Chem. Res. 1973, 6, 113–117;
- 16bE. H. Appelman, O. Dunkelberg, M. Kol, J. Fluorine Chem. 1992, 56, 199–213.
- 17L. M. Dennis, E. G. Rochow, J. Am. Chem. Soc. 1932, 54, 832–833;
- 17bL. M. Dennis, E. G. Rochow, J. Am. Chem. Soc. 1933, 55, 2431–2434.
- 18P. N. Noble, G. C. Pimentel, Spectrochim. Acta Part. A 1968, 24, 797–806.
- 19R. C. Thomson, E. H. Appelman, J. C. Sullivan, Inorg. Chem. 1977, 16, 2921–2926.
- 20S. Rozen, M. Brand, Angew. Chem. 1986, 98, 565; Angew. Chem. Int. Ed. Engl. 1986, 25, 554–555.
- 21M. B. Smith, J. March, March's Advanced Organic Chemistry, 5th ed., Wiley, New York, 2001, p. 76.
- 22R. Ballini, E. Marcatoni, M. Petrini, Tetrahedron Lett. 1992, 33, 4835–4838.
- 23G. A. Olah, P. Ramaiah, C.-S. Lee, G. K. S. Prakash, Synlett 1992, 337–339.
- 24
- 24a Houben-Weyl: Methoden der Organischen Chemie, Vol. 10/3 (Ed.: ), Thieme, Stuttgart, 1965, Houben-Weyl: Methoden der Organischen Chemie, Vol. E16A/2 (Ed.: ), Thieme, Stuttgart, 1990;
- 24bThe Chemistry of the Azide Group (Ed.: S. Patai), Wiley Interscience, New York, 1971 and Supplement D, Parts 1, 2, 1983;
- 24c Azides and Nitrenes (Ed.: ), Academic Press, Orlando, Florida, 1984;
- 24d The Chemistry of Halides, Pseudo-Halides, and Azides (Eds.: ), Wiley Interscience, New York, 1995, Parts 1 and 2;
- 24eG. K. S. Prakash, J. J. Struckhoff, K. Weber, A. Schreiber, B. Bau, G. A. Olah, J. Org. Chem. 1997, 62, 1872–1879.
- 25V. V. Rostovtsev, L. G. Green, V. V. Fokin, K. B. Sharpless, Angew. Chem. 2002, 114, 2708–2711;
10.1002/1521-3757(20020715)114:14<2708::AID-ANGE2708>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 2596–2599.10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar