Noncoordinating Anions—Fact or Fiction? A Survey of Likely Candidates
Ingo Krossing Priv.-Doz. Dr.
University of Karlsruhe, Engesserstrasse Geb. 30.45, 76128 Karlsruhe, Germany, Fax: (+49) 721-608-4854
Search for more papers by this authorInes Raabe Dipl.-Chem.
University of Karlsruhe, Engesserstrasse Geb. 30.45, 76128 Karlsruhe, Germany, Fax: (+49) 721-608-4854
Search for more papers by this authorIngo Krossing Priv.-Doz. Dr.
University of Karlsruhe, Engesserstrasse Geb. 30.45, 76128 Karlsruhe, Germany, Fax: (+49) 721-608-4854
Search for more papers by this authorInes Raabe Dipl.-Chem.
University of Karlsruhe, Engesserstrasse Geb. 30.45, 76128 Karlsruhe, Germany, Fax: (+49) 721-608-4854
Search for more papers by this authorGraphical Abstract
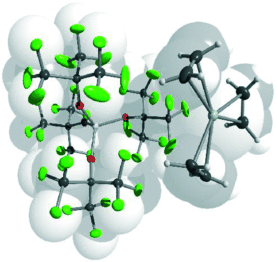
Weak but not meek: Weakly coordinating anions (WCAs) are capable of the stabilization of sensitive cations in condensed phases (see structure of [Ag(η2-C2H4)3]+[Al{OC(CF3)3}4]−). In this Review the latest developments with respect to fundemental and applied chemistry are summarized and the criteria for choosing the most suitable WCA for a particular system are explored.
Abstract
Is there anything resembling a truly noncoordinating anion? Would it not be great to be able to prepare any crazy, beautiful, or simply useful cationic species that one has in mind, or has detected by mass spectroscopy? In condensed phases the target cation has to be partnered with a suitable counteranion. This is the moment when difficulties arise and many wonderful ideas end in the sink owing to coordination or decomposition of the anion. However, maybe these counteranion problems can be overcome by one of the new weakly coordinating anions (WCAs). Herein is an overview on the available candidates in the quest for the least coordinating anion and a summary of new applications, available starting materials, and general strategies to introduce a WCA into a system. Some of the unusual properties of WCA salts such as high solubility in low dielectric media, pseudo gas-phase conditions in condensed phases, and the stabilization of weakly bound and low-charged complexes are rationalized on thermodynamic grounds. Limits of the WCAs, that is, anion coordination and decomposition, are shown and a quantum chemical analysis of all types of WCAs is presented which allows the choice of a particular WCA to be based on quantative data from a wide range of different anions.
References
- 1W. Beck, K. H. Sünkel, Chem. Rev. 1988, 88, 1405.
- 2Reviews:
- 2aC. Reed, Acc. Chem. Res. 1998, 31, 133;
- 2bS. H. Strauss, Chem. Rev. 1993, 93, 927.
- 3E. Y.-X. Chen, T. J. Marks, Chem. Rev. 2000, 100, 1391, and references therein.
- 4
- 4aK. Seppelt, Angew. Chem. 1993, 105, 1074; Angew. Chem. Int. Ed. Engl. 1993, 32, 1025;
- 4bM. Bochmann, Angew. Chem. 1992, 104, 1206; Angew. Chem. Int. Ed. Engl. 1992, 104, 1181.
- 5A. J. Lupinetti, S. H. Strauss, Chemtracts: Inorg. Chem. 1998, 11, 565.
- 6A. G. Massey, A. J. Park, J. Organomet. Chem. 1964, 2, 245.
- 7
- 7aJ. H. Golden, P. F. Mutolo, E. B. Lobrovski, F. J. DiSalvo, Inorg. Chem. 1994, 33, 5374;
- 7bK. Fujiki, S. Ikeda, H. Kobayashi, A. Mori, A. Nagira, J. Nie, T. Sonoda, Y. Yagupolskii, Chem. Lett. 2000, 66.
- 8J. van den Broeke, B.-J. Deelman, G. van Koten, Tetrahedron Lett. 2001, 42, 8085.
- 9K. Fujiki, J. Ichikawa, H. Kobayashi, A. Sonoda, T. Sonoda, J. Fluorine Chem. 2000, 102, 293.
- 10[B{C6F4(4-CF3)}4]−: F. A. R. Kaul, G. T. Puchta, H. Schneider, M. Grosche, D. Mihalios, W. A. Herrmann, J. Organomet. Chem. 2001, 621, 177.
- 11
- 11aL. Jia, X. Jang, C. L. Stern, T. J. Marks, Organometallics 1997, 16, 842;
- 11bL. Jia, X. Yang, A. Ishihara, T. J. Marks, Organometallics 1995, 14, 3135.
- 12G. Rodriguez, P. Brant, Organometallics 2001, 20, 2417.
- 13Most of those were unpublished results for the T. J. Marks group: See page 1398–1399 and references [78a,91,92] in ref. [3].
- 14One exception: The crystallization of the cyclotrigermenium salt [(GeSitBu3)3]+: A. Sekiguchi, N. Fukaya, M. Ichinobe, Y. Ishida, Eur. J. Inorg. Chem. 2000, 1155.
10.1002/(SICI)1099-0682(200006)2000:6<1155::AID-EJIC1155>3.3.CO;2-3 CAS Web of Science® Google Scholar
- 15
- 15aP. Biagini, G. Luigli, L. Abis, P. Andeussi (Enichem), US Patent 5 602 269, 1997;
- 15bM. Bochmann, M. J. Sarsfield, Organometallics 1998, 17, 5908.
- 16K. Ren, A. Mejiritski, J. H. Malpert, D. C. Neckers, Tetrahedron Lett. 2000, 41, 8669.
- 17K. Ren, J. H. Malpert, H. Li, H. Gu, D. C. Neckers, Macromolecules 2002, 35, 1632.
- 18
- 18aJ. Zhou, S. J. Lancaster, D. A. Walker, S. Beck, M. Thornton-Pett, M. Bochmann, J. Am. Chem. Soc. 2001, 123, 223;
- 18bS. J. Lancaster, D. A. Walker, S. Beck, M. Thornton-Pett, M. Bochmann, Chem. Commun. 1999, 1533;
- 18cR. E. LaPointe, WO 99/42467, 1999.
- 19D. Vagedes, G. Erker, R. Fröhlich, J. Organomet. Chem. 2002, 641, 148.
- 20R. E. LaPointe, G. R. Roof, K. A. Abboud, J. Klosin, J. Am. Chem. Soc. 2000, 122, 9560.
- 21S. J. Lancaster, A. Rodriguez, A. Lara-Sanchez, M. D. Hannant, D. A. Walker, D. H. Hughes, M. Bochmann, Organometallics 2002, 21, 451.
- 22V. C. Williams, W. E. Piers, W. Clegg, S. Collins, T. B. Marder, J. Am. Chem. Soc. 1999, 121, 3244.
- 23L. D. Henderson, W. E. Piers, G. J. Irvine, R. McDonald, Organometallics 2002, 21, 340.
- 24M. Schmidt, A. Kühner, E. Bernhardt, H. Willner, EP 1205480A2, 2002.
- 25E. Bernhardt, G. Henkel, H. Willner, G. Pawelke, H. Bürger, Chem. Eur. J. 2001, 7, 4696.
10.1002/1521-3765(20011105)7:21<4696::AID-CHEM4696>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 26R. D. Chambers, H. C. Clark, C. J. Willis, J. Am. Chem. Soc. 1960, 82, 5298.
- 27M. Schmidt, A. Kühner, K.-D. Franz, G.-V. Röschenthaler, DE 101 03 189A1, 2002.
- 28E. Bernhardt, M. Finze, C. W. Lehmann, F. Aubke, H. Willner Angew. Chem. 2003, 115, 2123;
10.1002/ange.200250780 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 2071.
- 29D. J. Brauer, H. Bürger, Y. Chebude, G. Pawelke, Inorg. Chem. 1999, 38, 3972.
- 30For a recent review on [(CF3)3BY]− compounds see: G. Pawelke, H. Bürger, Coord. Chem. Rev. 2001, 215, 243.
- 31M. Finze, E. Bernhardt, A. Terheiden, M. Berkei, H. Willner, D. Christen, H. Oberhammer, F. Aubke, J. Am. Chem. Soc. 2002, 124, 15 385.
- 32
- 32aV. V. Bardin, H. J. Frohn, Main Group Met. Chem. 2002, 25, 589;
- 32bH. J. Frohn, N. Y. Adonin, V. V. Bardin, V. F. Starichenko, J. Fluorine Chem. 2002, 117, 115;
- 32cV. V. Bardin, S. G. Idemskaya, H. J. Frohn, Z. Anorg. Allg. Chem. 2002, 628, 883.
- 33J. Plesek, T. Jelinek, S. Hermanek, B. Stíbr, Collect. Czech. Chem. Commun. 1986, 51, 819.
- 34
- 34aC. A. Reed, K.-C. Kim, E. S. Stoyanov, D. Stasko, F. S. Tham, L. J. Mueller, P. D. W. Boyd, J. Am. Chem. Soc. 2003, 125, 1796;
- 34bC. A. Reed, K.-C. Chan, R. D. Bolskar, L. J. Mueller, Science 2000, 289, 101;
- 34cC. A. Reed, N. L. P. Fackler, K.-C. Kim, D. Stasko, D. R. Evans, L. J. Mueller, P. D. W. Boyd, C. E. F. Rickard, J. Am. Chem. Soc. 1999, 121, 6314;
- 34dD. Stasko, C. A. Reed, J. Am. Chem. Soc. 2002, 124, 1148.
- 35K.-C. Kim, C. A. Reed, D. W. Elliott, L. J. Mueller, F. Tham, L. Lin, J. B. Lambert, Science 2002, 297, 825.
- 36C. A. Reed, K.-C. Kim, R. D. Bolskar, L. J. Mueller, Science 2000, 289, 101.
- 37K.-C. Kim, C. A. Reed, G. S. Long, A. Sen, J. Am. Chem. Soc. 2002, 124, 7662.
- 38
- 38aC.-W. Tsang, Q. Yang, E. Tung-Po Sze, T. C. W. Mak, D. T. W. Chan, Z. Xie, Inorg. Chem. 2000, 39, 5851;
- 38bZ. Xie, C.-W. Tsang, E. Tung-Po Sze, Q. Yang, D. T. W. Chan, T. C. W. Mak, Inorg. Chem. 1998, 37, 6444.
- 39Z. Xie, C.-W. Tsang, F. Xue, T. C. W. Mak, J. Organomet. Chem. 1999, 577, 197.
- 40B. T. King, Z. Janousek, B. Grüner, M. Tramell, B. C. Noll, J. Michl, J. Am. Chem. Soc. 1996, 118, 3313.
- 41B. T. King, J. Michl, J. Am. Chem. Soc. 2000, 122, 10 255.
- 42S. M. Ivanova, S. V. Ivanov, S. M. Miller, O. P. Anderson, K. A. Solntsev, S. H. Strauss, Inorg. Chem. 1999, 38, 3756.
- 43
- 43aS. V. Ivanov, J. J. Rockwell, O. G. Polyakov, C. M. Gaudinski, O. P. Anderson, K. A. Solntsev, S. H. Strauss, J. Am. Chem. Soc. 1998, 120, 4224;
- 43bS. H. Strauss in Contemporary Boron Chemistry (Ed.: ), Royal Society of Chemistry, Cambridge, 2000, P. 44.
- 44C.-W. Tsang, Q. Yang, E. Tung-Po Sze, T. C. W. Mak, D. T. W. Chan, Z. Xie, Inorg. Chem. 2000, 39, 3582.
- 45S. V. Ivanov, S. M. Miller, O. P. Anderson, K. A. Solntsev, S. H. Strauss, J. Am. Chem. Soc. 2003, 125, 4694.
- 46
- 71A. Franken, B. T. King, J. Rudolph, P. Rao, B. C. Noll, J. Michl, Collect. Czech. Chem. Commun. 2001, 66, 1238;
- 46bA. Franken, B. T. King, J. Michl, WO 2002079210, 2002.
- 47I. Krossing, Chem. Eur. J. 2001, 7, 490.
10.1002/1521-3765(20010119)7:2<490::AID-CHEM490>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 48[Al(ORF)4]−:
- 48aT. J. Barbarich, S. T. Handy, S. M. Miller, O. P. Anderson, P. A. Grieco, S. H. Strauss, Organometallics 1996, 15, 3776;
- 48bT. J. Barbarich, S. M. Miller, O. P. Anderson, S. H. Strauss, J. Mol. Catal. A 1998, 128, 289;
- 48cS. M. Ivanova, B. G. Nolan, Y. Kobayashi, S. M. Miller, O. P. Anderson, S. H. Strauss, Chem. Eur. J. 2001, 7, 503.
10.1002/1521-3765(20010119)7:2<503::AID-CHEM503>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 49S. H. Strauss, B. G. Nolan, B. P. Fauber, WO 00/53611, 2000.
- 50M. Gonsior, I. Krossing, N. Mitzel, Z. Anorg. Allg. Chem. 2002, 628, 1821.
- 51I. Krossing, H. Brands, R. Feuerhake, S. Koenig, J. Fluorine Chem. 2001, 112, 83.
- 52
- 52aY. Sun, M. V. Metz, C. L. Stern, T. J. Marks, Organometallics 2000, 19, 1625;
- 52bM. V. Metz, Y. Sun, C. L. Stern, T. J. Marks, Organometallics 2002, 21, 3691.
- 53F. A. R. Kaul, G. T. Puchta, H. Schneider, M. Grosche, D. Mihalios, W. A. Herrmann, J. Organomet. Chem. 2001, 621, 184.
- 54By scaling up the procedure given in ref. [47]. Note added in proof (March 30, 2004): This Li salt is now available from Strem.
- 55
- 55aA. Reisinger, I. Krossing, Diplomarbeit, Universität Karlsruhe 2003;
- 55bI. Krossing, A. Reisinger, Angew. Chem. 2003, 115, 5903; Angew. Chem. Int. Ed. 2003, 42, 5725.
- 56
- 56aI. Krossing, J. Am. Chem. Soc. 2001, 123, 4603;
- 56bI. Krossing, L. van Wüllen, Chem. Eur. J. 2002, 8, 700.
10.1002/1521-3765(20020201)8:3<700::AID-CHEM700>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 57A. Adolf, M. Gonsior, I. Krossing, J. Am. Chem. Soc. 2002, 124, 7111.
- 58T. S. Cameron, A. Decken, I. Dionne, Min Fang, I. Krossing, J. Passmore, Chem. Eur. J. 2002, 8, 3386.
10.1002/1521-3765(20020802)8:15<3386::AID-CHEM3386>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 59
- 59aI. Krossing, I. Raabe, Angew. Chem. 2001, 113, 4544;
Angew. Chem. Int. Ed. 2001, 40, 4406;
10.1002/1521-3773(20011203)40:23<4406::AID-ANIE4406>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 59bM. Gonsior, I. Krossing, L. Müller, I. Raabe, M. Jansen, L. van Wüllen, Chem. Eur. J. 2002, 8, 4475.
10.1002/1521-3765(20021004)8:19<4475::AID-CHEM4475>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 60I. Krossing, A. Bihlmeier, I. Raabe, N. Trapp, Angew. Chem. 2003, 115, 1569;
10.1002/ange.200250172 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1531.
- 61M. Bochmann, I. Krossing, unpublished results. [Ph3C]+[Al{OC(CF3)3}4]− was used as an activator for ethene and propene polymerization with [(SBI)ZrMe2] in the presence of AliBu3. It was found that the aluminate was an equally efficient initiator as [Ph3C]+[B(C6F5)4]−.
- 62D. M. Van Seggan, P. K. Hurtburt, M. D. Noirot, O. P. Anderson, S. H. Strauss, Inorg. Chem. 1992, 31, 1423.
- 63[E(OTeF5)6]− (E=As, Sb, Bi): H. P. A. Mercier, J. C. P. Saunders, G. T. Schrobilgen, J. Am. Chem. Soc. 1994, 116, 2921.
- 64[E(OTeF5)6]− (E=Sb, Nb): D. M. Van Seggen, P. K. Hurlburt, O. P. Anderson, S. H. Strauss, Inorg. Chem. 1995, 34, 3453.
- 65[Sb(OTeF5)6]−: T. S. Cameron, I. Krossing, J. Passmore, Inorg. Chem. 2001, 40, 2001.
- 66K. Moock, K. Seppelt, Z. Anorg. Allg. Chem. 1988, 561, 132.
- 67P. K. Hurlburt, J. J. Rack, J. S. Luck, S. F. Dec, J. D. Webb, O. P. Anderson, S. H. Strauss, J. Am. Chem. Soc. 1994, 116, 10 003.
- 68M. Gerken, P. Kolb, A. Wegner, H. P. A. Mercier, H. Borrmann, D. A. Dixon, G. J. Schrobilgen, Inorg. Chem. 2000, 39, 2813.
- 69W. J. Casteel, Jr.,P. Kolb, N. LeBlond, H. P. A. Mercier, G. J. Schrobilgen, Inorg. Chem. 1996, 35, 929.
- 70G. J. Schrobilgen, presented at the ACS meeting in New Orleans, March 2003, INOR 770.
- 71T. S. Cameron, I. Dionne, I. Krossing, J. Passmore, Solid State Sci. 2002, 4, 1435.
- 72
- 72aS. Brownstein, Can. J. Chem. 1969, 47, 605;
- 72bK. O. Christe, W. Maya, Inorg. Chem. 1969, 8, 1253;
- 72cP. A. W. Dean, R. J. Gillespie, R. Hume, J. Chem. Soc. D 1969, 990.
- 73
- 73aR. Minkwitz, F. Neikes, Inorg. Chem. 1999, 38, 5960;
- 73bR. Minkwitz, C. Hirsch, Eur. J. Inorg. Chem. 1999, 2249;
10.1002/(SICI)1099-0682(199912)1999:12<2249::AID-EJIC2249>3.0.CO;2-1 CAS Web of Science® Google Scholar
- 73cK. O. Christe, X. Zhang, R. Bau, J. Hegge, G. A. Olah, G. K. S. Prakash, J. A. Sheehy, J. Am. Chem. Soc. 2000, 122, 481.
- 74[Sb3F16]−:
- 74aI. Bernhardi, T. Drews, K. Seppelt, Angew. Chem. 1999, 111, 2370;
10.1002/(SICI)1521-3757(19990802)111:15<2370::AID-ANGE2370>3.0.CO;2-H Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2232;10.1002/(SICI)1521-3773(19990802)38:15<2232::AID-ANIE2232>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 74bR. Faggiani, D. K. Kennepohl, C. J. L. Lock, G. J. Schrobilgen, Inorg. Chem. 1986, 25, 563;
- 74cT. Drews, W. Koch, K. Seppelt, J. Am. Chem. Soc. 1999, 121, 4379.
- 75[Sb4F21]−: T. Drews, K. Seppelt, Angew. Chem. 1997, 109, 264;
10.1002/ange.19971090318 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 273.
- 76A. J. Edwards, G. R. Jones, R. J. Sills, J. Chem. Soc. Chem. Commun. 1968, 1527.
- 77S. Seidel, K. Seppelt, Science 2000, 290, 117.
- 78T. Drews, S. Seidel, K. Seppelt, Angew. Chem. 2002, 114, 470;
10.1002/1521-3757(20020201)114:3<470::AID-ANGE470>3.0.CO;2-U Google ScholarAngew. Chem. Int. Ed. 2002, 41, 454.10.1002/1521-3773(20020201)41:3<454::AID-ANIE454>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar
- 79
- 79aH. Willner, M. Bodenbinder, R. Bröchler, G. Hwang, S. J. Rettig, J. Trotter, B. von Ahsen, U. Westphal, V. Jonas, W. Thiel, F. Aubke, J. Am. Chem. Soc. 2001, 123, 588;
- 79bH. Willner, F. Aubke, Angew. Chem. 1997, 109, 2506; Angew. Chem. Int. Ed. Engl. 1997, 36, 2402;
- 79cH. Willner, F. Aubke, Inorg. Chem. Highlights 2002, 195.
- 80
- 80aH. D. B. Jenkins, H. K. Roobottom, J. Passmore, L. Glasser, Inorg. Chem. 1999, 38, 3609;
- 80bT. S. Cameron, R. J. Deeth, I. Dionne, H. Du, H. D. B. Jenkins, I. Krossing, J. Passmore, H. K. Roobottom, Inorg. Chem. 2000, 39, 5614;
- 80cS. Brownridge, H. D. B. Jenkins, I. Krossing, J. Passmore, H. K. Roobottom, Coord. Chem. Rev. 2000, 197, 397;
- 80dT. E. Mallouk, G. L. Rosenthal, G. Muller, R. Brusasco, N. Bartlett, Inorg. Chem. 1984, 23, 3167.
- 81K. O. Christe, D. A. Dixon, D. McLemore, W. W. Wilson, J. Sheehy, J. A. Bootz, J. Fluorine Chem. 2000, 101, 151.
- 82I.-C. Hwang, K. Seppelt, Angew. Chem. 2001, 113, 3803;
Angew. Chem. Int. Ed. 2001, 40, 3690.
10.1002/1521-3773(20011001)40:19<3690::AID-ANIE3690>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 83Reviews: ref. [3] and
- 83aW. E. Piers, T. Chivers, Chem. Soc. Rev. 1997, 26, 345;
- 83bW. E. Piers, G. J. Irvine, V. C. Williams, Eur. J. Inorg. Chem. 2000, 2131;
- 83cG. Erker, Acc. Chem. Res. 2001, 34, 309;
- 83dF. P. Gabbaï, Angew. Chem. 2003, 115, 2318; Angew. Chem. Int. Ed. 2003, 42, 2218; For related Lewis acids based on polyfluoroaromatic metalloids see:
- 83eG. M. Brooke, J. Fluorine Chem. 1997, 86, 1;
- 83fS. C. Cohen, A. G. Massey, Adv. Fluorine Chem. 1970, 6, 83.
- 84A. G. Massey, J. Park, J. Organomet. Chem. 1966, 5, 218.
- 85
- 85aX. Yang, C. L. Stern, T. J. Marks, J. Am. Chem. Soc. 1991, 113, 3623;
- 85bX. Yang, C. L. Stern, T. J. Marks, J. Am. Chem. Soc. 1994, 116, 10 015.
- 86J. A. Ewen, M. J. Elder, Eur. Patent Appl. 0 427 697, 1991; J. A. Ewen, M. J. Elder, US Patent 5 561 092, 1996.
- 87H. Jacobsen, H. Berke, S. Döring, G. Kehr, G. Erker, R. Fröhlich, O. Meyer, Organometallics 1999, 18, 1724.
- 88
- 88aY. X. Chen, C. L. Stern, S. Yang, T. J. Marks, J. Am. Chem. Soc. 1996, 118, 12 451;
- 88bL. Li, C. L. Stern, T. J. Marks, Organometallics 2000, 19, 3332.
- 89
- 89aL. Li, T. J. Marks, Organometallics 1998, 17, 3996;
- 89bT. J. Marks, L. Li, Y. X. Chen, M. H. McAdon, P. N. Nickias, WO WO99/06412, 1999.
- 90
- 90aV. C. Williams, W. E. Piers, W. Clegg, S. Collins, T. B. Marder, J. Am. Chem. Soc. 1999, 121, 3244;
- 90bV. C. Williams, G. J. Irvine, W. E. Piers, Z. Li, S. Collins, W. Clegg, M. R. J. Elsegood, T. B. Marder, Organometallics 2000, 19, 1619.
- 91C. Fritze, F. Küber, H. Böhmen (Hoechst AG), Eur. Patent Appl. 811 627, 1997.
- 92
- 92aV. C. Williams, C. Dai, Z. Li, S. Collins, W. E. Piers, W. Clegg, M. R. J. Elsegood, T. B. Marder, Angew. Chem. 1999, 111, 3922;
10.1002/(SICI)1521-3757(19991216)111:24<3922::AID-ANGE3922>3.0.CO;2-G Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3695;10.1002/(SICI)1521-3773(19991216)38:24<3695::AID-ANIE3695>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 92bM. V. Metz, D. J. Schwartz, C. L. Stern, P. N. Nickias, T. J. Marks, Angew. Chem. 2000, 112, 1368;
10.1002/(SICI)1521-3757(20000403)112:7<1368::AID-ANGE1368>3.0.CO;2-H Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1312;
- 92cM. H. McAdon, P. N. Nickias, T. J. Marks, WO WO99/06413, 1999.
- 93G. Kehr, R. Fröhlich, B. Wibbeling, G. Erker, Chem. Eur. J. 2000, 6, 258.
10.1002/(SICI)1521-3765(20000117)6:2<258::AID-CHEM258>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 94
- 94aH.-H. Brintzinger, D. Fischer, R. Mülhaupt, B. Rieger, R. Waymouth, Angew. Chem. 1995, 107, 1255;
10.1002/ange.19951071104 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1143;
- 94bW. E. Piers, Chem. Eur. J. 1998, 4, 13.
- 95
- 95aS. D. Ittel, L. K. Johnson, M. Brookhart, Chem. Rev. 2000, 100, 1169;
- 95bG. J. P. Britovsek, V. C. Gibson, D. F. Wass, Angew. Chem. 1999, 111, 448;
10.1002/(SICI)1521-3757(19990215)111:4<448::AID-ANGE448>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 1999, 38, 429;
- 95cS. Mecking, Coord. Chem. Rev. 2000, 203, 305.
- 96
- 96aR. Braun, J. Sauer, Chem. Ber. 1986, 119, 1269;
- 96bP. A. Grieco, J. J. Nunes, M. D. Gaul, J. Am. Chem. Soc. 1990, 112, 4595;
- 96cA. Flohr, H. Waldmann, J. Prakt. Chem. 1995, 337, 609.
- 97Reviews:
- 97aA. Kumar, Chem. Rev. 2001, 101, 1;
- 97bS. Saito in Lewis Acids in Organic Synthesis, Vol. 1 (Ed.: ), Wiley-VCH, Weinheim, 2000, p. 9.
- 98S. Moss, B. T. King, A. de Meijere, S. I. Kozushkov, P. E. Eaton, J. Michl, Org. Lett. 2001, 3, 2001.
- 99K. Fujiki, S. Ikeda, H. Kobayashi, A. Mori, A. Nagira, J. Nie, T. Sonoda, Y. Yagupolskii, Chem. Lett. 2000, 66.
- 100[Nb(ORF)6]−: G. M. Kloster, W. J. Dubaz, P. A. Grieco, D. F. Shriver, S. H. Strauss, Inorg. Chim. Acta 1997, 263, 195.
- 101
- 101aP. A. Grieco, W. J. DuBay, L. J. Todd, Tetrahedron Lett. 1996, 37, 8707;
- 101bW. J. DuBay, P. A. Grieco, L. J. Todd, J. Org. Chem. 1994, 59, 6898.
- 102N. J. Patmore, C. Hague, J. H. Cotgreave, M. F. Mahon, C. G. Frost, A. S. Weller, Chem. Eur. J. 2002, 8, 2088.
10.1002/1521-3765(20020503)8:9<2088::AID-CHEM2088>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 103See, for example:
- 103aN. J. Stone, D. A. Sweigart, A. M. Bond, Organometallics 1986, 5, 2553;
- 103bC. G. Zoski, D. A. Sweigart, N. J. Stone, P. H. Rieger, E. Mocellin, T. F. Mann, D. R. Mann, D. K. Gosser, M. M. Doeff, A. M. Bond, J. Am. Chem. Soc. 1988, 110, 2109.
- 104See, for example:
- 104aR. Rulkens, A. J. Lough, I. Manners, S. R. Lovelace, C. Grant, W. E. Geiger, J. Am. Chem. Soc. 1996, 118, 12 683;
- 104bB. Grossmann, J. Heinze, E. Herdtweck, F. H. Köhler, H. Nöth, H. Schwenk, M. Spiegler, W. Wachter, B. Weber, Angew. Chem. 1997, 109, 384;
10.1002/ange.19971090416 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 387.
- 105
- 105aR. J. LeSuer, W. E. Geiger, Angew. Chem. 2000, 112, 254;
10.1002/(SICI)1521-3757(20000103)112:1<254::AID-ANGE254>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 2000, 39, 248;10.1002/(SICI)1521-3773(20000103)39:1<248::AID-ANIE248>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 105bF. Barriere, N. Camire, W. E. Geiger, U. T. Mueller-Westerhoff, R. Sanders, J. Am. Chem. Soc. 2002, 124, 7262;
- 105cN. Camire, A. Nafady, W. E. Geiger, J. Am. Chem. Soc. 2002, 124, 7260;
- 105dN. Camire, U. T. Mueller-Westerhoff, W. E. Geiger, J. Organomet. Chem. 2001, 637–639, 823.
- 106
- 106aP. G. Gassman, P. A. Deck, Organometallics 1994, 13, 1934;
- 106bM. G. Hill, W. M. Lamanna, K. R. Mann, Inorg. Chem. 1991, 30, 4687;
- 106cP. G. Gassman, J. R. Sowa, Jr.,M. G. Hill, K. R. Mann, Organometallics 1995, 14, 4879.
- 107L. Pospisil, B. T. King, J. Michl, Electrochim. Acta 1998, 44, 103.
- 108http://www.ionicliquids-merck.de.
- 109Reviews:
- 109aP. Wasserscheid, W. Keim, Angew. Chem. 2000, 112, 3927;
10.1002/1521-3757(20001103)112:21<3926::AID-ANGE3926>3.0.CO;2-U Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3772;10.1002/1521-3773(20001103)39:21<3772::AID-ANIE3772>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 109bJ. D. Holbrey, Clean Prod. Processes 1999, 1, 2071;
- 109cT. Welton, Chem. Rev. 1999, 99, 2071;
- 109dP. Wasserscheid, Chem. Unserer Zeit 2003, 37, 52.
- 110A. Bösmann, G. Francio, E. Janssen, W. Leitner, P. Wasserscheid, Angew. Chem. 2001, 113, 2769;
10.1002/1521-3757(20010716)113:14<2769::AID-ANGE2769>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2697.10.1002/1521-3773(20010716)40:14<2697::AID-ANIE2697>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 111A. S. Larsen, J. D. Holbrey, F. S. Tham, C. A. Reed, J. Am. Chem. Soc. 2000, 122, 7264.
- 112See also the related straight forward synthesis of K+[F7C3BF3]− and K+[F13C6BF3]−: H.-J. Frohn, V. V. Bardin, Z. Anorg. Allg. Chem. 2001, 627, 15.
- 113
- 113aF. Kita, H. Sakata, A. Kawakami, H. Kamizori, T. Sonoda, H. Nagashima, N. V. Pavlenko, Y. Yagupolskii, J. Power Sources 2001, 97–98, 581;
- 113bL. M. Yagupolski, L. Yu, J. Yagupolski, J. Fluorine Chem. 1995, 72, 225;
- 113cN. Ignat'tev, P. Sartori, J. Fluorine Chem. 2001, 101, 203.
- 114W. Xu, C. A. Austen, Electrochem. Solid-State Lett. 2000, 3, 366.
- 115F. Kita, H. Sakata, S. Sinomoto, A. Kawakami, H. Kamizori, T. Sonoda, H. Nagashima, J. Nie, N. V. Pavlenko, Y. Yagupolskii, J. Power Sources 2000, 90, 27.
- 116H. Suzuki, H. Naganawa, S. Tachimori, Phys. Chem. Chem. Phys. 2003, 5, 726.
- 117J. V. Crivello, Radiat. Curing Polym. Sci. Technol. 1993, 2, 435.
- 118F. Castellanos, J. P. Fouassier, C. Priou, J. Cavezzan, J. Appl. Polym. Sci. 1996, 60, 705.
- 119
- 119aK. Ren, A. Mejiritski, J. H. Malpert, O. Grinevich, H. Gu, D. C. Neckers, Tetrahedron Lett. 2000, 41, 8669;
- 119bH. Li, K. Ren, W. Zhang, J. H. Malpert, D. C. Neckers, Macromolecules 2001, 34, 4161;
- 119cH. Gu, K. Ren, A. Mejiritski, O. Grinevich, J. H. Malpert, D. C. Neckers, J. Org. Chem. 2001, 66, 4161.
- 120K. Ren, J. H. Malpert, H. Li, H. Gu, D. C. Neckers, Macromolecules 2002, 35, 1632.
- 121H. Nishida, N. Takada, M. Yoshimura, T. Sonoda, H. Kobayashi, Bull. Chem. Soc. Jpn. 1984, 57, 2600.
- 122Further purification of Na+[B(C6H3(CF3)2)4]−:
- 122aA. D. Hennis, J. D. Polley, G. S. Long, A. Sen, D. Yandulov, J. Lipian, G. M. Benedikt, L. F. Rhodes, J. C. Huffman, Organometallics 2001, 20, 2802;
- 122bR. Taube, S. Wache, J. Organomet. Chem. 1992, 428, 431.
- 123
- 123aJ. C. W. Chien, W.-M. Tsai, M. D: Rausch, J. Am. Chem. Soc. 1991, 113, 8570;
- 123bJ. A. Ewen, M. J. Elder, Eur. Patent Appl. 0 426 637, 1991; M. Bochmann, S. J. Lancaster, J. Organomet. Chem. 1992, 434, C 1.
- 124
- 124aZ. Xie, B.-M. Wu, T. C. W. Mak, J. Manning, C. A. Reed, J. Chem. Soc. Dalton Trans. 1997, 1213;
- 124bZ. Xie, J. Manning, R. W. Reed, R. Mathur, P. D. W. Boyd, A. Benesi, C. A. Reed, J. Am. Chem. Soc. 1996, 118, 2922;
- 124cZ. Xie, T. Jelinek, R. Bau, C. A. Reed, J. Am. Chem. Soc. 1994, 116, 1907;
- 124dK. Shelly, C. A. Reed, Y. J. Lee, W. R. Scheidt, J. Am. Chem. Soc. 1986, 108, 3117;
- 124eD. J. Liston, Y. J. Lee, W. R. Scheidt, C. A. Reed, J. Am. Chem. Soc. 1989, 111, 6643.
- 125
- 125aB. T. King, B. C. Noll, J. Michl, Collect. Czech. Chem. Commun. 1999, 64, 1001;
- 125bB. T. King, Z. Janusek, J. Michl, US 5731470 A, 1998;
- 125cB. T. King, Z. Janusek, J. Michl, WO 9631519A1, 1996.
- 126S. H. Strauss, S. V. Ivanov, WO 2002036557, 2002.
- 127S. H. Strauss, S. V. Ivanov, WO 98/43983, 1998.
- 128
- 128aM. Brookhart, B. Grant, A. F. Volpe, Jr., Organometallics 1992, 11, 3920;
- 128bD. J. Tempel, L. K. Johnson, R. L. Huff, P. S. White, M. Brookhart, J. Am. Chem. Soc. 2000, 122, 6686.
- 129K. Fujiki, M. Kashiwagi, H. Miyamoto, A. Sonoda, J. Ichikawa, H. Kobayashi, T. Sonoda, J. Fluorine Chem. 1992, 57, 307.
- 130P. Jutzi, C. Müller, A. Stammler, H.-G. Stammler, Organometallics 2000, 19, 1442.
- 131
- 131aG. G. Hladky, D. J. Upton, H. W. Turner, WO 91/09882, 1992;
- 131bX. Yang, C. L. Stern, T. J. Marks, Organometallics 1991, 10, 840;
- 131cH. W. Turner, EP 0 277 004 A1, 1988.
- 132D. Stasko, S. P. Hoffmann, K.-C. Kim, N. L. P. Fackler, A. S. Larsen, T. Drovetskaya, F. S. Tham, C. A. Reed, C. E. F. Rickard, P. D. W. Boyd, E. S. Stoyanov, J. Am. Chem. Soc. 2002, 124, 13 869.
- 133C. W. Tsang, Q.-C. Yang, T. C. W. Mak, Z.-W. Xie, Chin. J. Chem. 2002, 20, 1241.
- 134Review: N. G. Conolly, W. E. Geiger, Chem. Rev. 1996, 96, 877.
- 135M. Gonsior, F. Breher, I. Krossing, unpublished results.
- 136R. D. Bolskar, R. S. Mathur, C. A. Reed, J. Am. Chem. Soc. 1998, 118, 13 093.
- 137B. T. King, B. C. Noll, A. J. McKinley, J. Am. Chem. Soc. 1996, 118, 10 902.
- 138I. Zharov, B. T. King, Z. Havlas, A. Pardi, J. Michl, J. Am. Chem. Soc. 2000, 122, 10 253.
- 139E. Y.-X. Chen, K. A. Abboud, Organometallics 2000, 19, 5541.
- 140Ref. [74a].
- 141
- 141aA. V. Korolev, E. Ihara, I. A. Guzei, V. G. Joung, Jr.,R. F. Jordan, J. Am. Chem. Soc. 2001, 123, 8291;
- 141bA. V. Korolev, F. Delpech, S. Dagorne, I. A. Guzei, R. F. Jordan, Organometallics 2001, 20, 3367;
- 141cF. Delpech, I. A. Guzei, R. F. Jordan, Organometallics 2002, 21, 1167.
- 142
- 142aH. C. Strauch, B. Wibbeling, R. Fröhlich, G. Erker, H. Jacobsen, H. Berke, Organometallics 1999, 18, 3802;
- 142bH. C. Strauch, G. Erker, R. Fröhlich, Organometallics 1998, 17, 5746.
- 143Übersichten:
- 143aJ. B. Lambert, L. Kania, S. Zhang, Chem. Rev. 1995, 95, 1191;
- 143bC. A. Reed, Acc. Chem. Res. 1998, 31, 325.
- 144S. Kainz, A. Brinkmann, W. Leitner, A. Pfaltz, J. Am. Chem. Soc. 1999, 121, 6421.
- 145A. J. Lupinetti, M. D. Havighurst, S. M. Miller, O. P. Anderson, S. H. Strauss, J. Am. Chem. Soc. 1999, 121, 11 920.
- 146
- 146aG. A. Olah, G. Rasul, L. Heiliger, G. K. S. Prakash, J. Am. Chem. Soc. 1996, 118, 3580;
- 146bG. A. Olah, L. Heiliger, G. K. S. Prakash, J. Am. Chem. Soc. 1989, 111, 8020;
- 146cH. Vancik, K. Percac, D. E. Sunko, J. Am. Chem. Soc. 1990, 112, 7418;
- 146dJ. W. Hudgens, R. D. Johnson III, B. P. Tsai, S. A. Kafafi, J. Am. Chem. Soc. 1990, 112, 5763;
- 146eG. A. Olah, Y. K. Mo, E. G. Melby, H. C. Lin, J. Org. Chem. 1973, 38, 367;
- 146fG. A. Olah, G. Rasul, A. K. Yudin, A. Burrichter, G. K. S. Prakash, A. L. Chistyakov, A. V. Stankevich, I. S. Akhrem, N. P. Gambaryan, M. E. Vol'pin, J. Am. Chem. Soc. 1996, 118, 1446.
- 147L. H. Shultz, M. Brookhart, Organometallics 2001, 20, 3975.
- 148M. D. Hannant, M. Schormann, M. Bochmann, Dalton Trans. 2002, 4071.
- 149G. Kehr, R. Roesmann, R. Fröhlich, C. Holst, G. Erker, Eur. J. Inorg. Chem. 2001, 535.
- 150A. Vij, W. W. Wilson, V. Vij, F. S. Tham, J. A. Sheehy, K. O. Christe, J. Am. Chem. Soc. 2001, 123, 6308.
- 151Ref. [74c].
- 152N. Wiberg, Coord. Chem. Rev. 1997, 163, 217, and references therein.
- 153B. Twamley, S. T. Haubrich, P. P. Power, Adv. Organomet. Chem. 1999, 44, 1.
- 154E. Lork, D. Viets, R. Mews, H. Oberhammer, Inorg. Chem. 2000, 39, 4838.
- 155A. R. Mahjoub, X. Zhang, K. Seppelt, Chem. Eur. J. 1995, 1, 261.
- 156A. Kolomeitsev, G. Bisky, E. Lork, V. Movchun, E. Rusanov, P. Kirsch, G.-V. Röschenthaler, Chem. Commun. 1999, 1017.
- 157M. Lin, C. Liu, L. Zheng, Wuli Huaxue Xuebao 1995, 11, 266.
- 158Ref. [80a].
- 159
- 159aD. D. Wagman, W. H. Evans, V. B. Parker, R. H. Schumm, I. Halow, S. M. Bailey, K. L. Churney, R. L. Nuttal, J. Phys. Chem. Ref. Data 1982, 11 (Suppl. 2);
- 159bS. G. Lias, J. E. Bartmess, J. F. Liebman, J. L. Holmes, R. D. Levin, W. G. Mallard, J. Phys. Chem. Ref. Data 1988, 17 (Suppl. 1);
- 159c CRC Handbook of Chemistry und Physics, CRC Press, ;
- 159dhttp://www.nist.gov/chemistry.
- 160The simple Born equation was used to approximate the Gibbs solvation energy: ΔGsolv.=0.5{z2 e Na/4πε0 r}{1−1/εrel.}. z=charge of the ion, e=charge of the electron, Na=Avogadro's constant, ε0=dielectric constant of the vacuum, r=ionic radius, εrel. relative dielectric constant of the medium. For the plot r was arbitrarily set to 2.0 Å.
- 161For the estimation of lattice versus Gibbs solvation energy effects the size of the cation A+ was arbitrarily set to a thermochemical volume of 10 Å3 equivalent to an ionic radius of about 1.3 Å (similar to that of K+). Then the volume (radius) of the ion X− was changed from 10 Å3 (1.3 Å) to 1250 Å3 (6.7 Å) and solvation as well as lattice enthalpies were calculated for A+X−. Gibbs energies of solvation were approximated by the Born equation,[160] lattice enthalpies by the volume based equation of Jenkins and Passmore.[158]
- 162H. W. Roesky, M. Thomas, J. Schimkowiak, P. G. Jones, W. Pinkert, G. M. Sheldrick, J. Chem. Soc. Chem. Commun. 1982, 895.
- 163A. J. Lupinetti, V. Jonas, W. Thiel, S. H. Strauss, G. Frenking, Chem. Eur. J. 1999, 5, 2573.
10.1002/(SICI)1521-3765(19990903)5:9<2573::AID-CHEM2573>3.0.CO;2-J CAS Web of Science® Google Scholar
- 164The thermochemical volume of [Al(OR)4]− is 758 Å3,[51] that of [Sb(OTeF5)6]− is 725 Å3.[65] Therefore the [B(OTeF5)4]− ion should constitute approximately 2/3 of the volume of the [Sb(OTeF5)6]− ion or about 483 Å3.
- 165See ref. [166] for an up to date overview.
- 166J. Kanetti, L. C. P. M. de Smet, R. Boom, H. Zuilhof, E. J. R. Sudhölter, J. Phys. Chem. A 2002, 106, 11 197–11 204.
- 167
- 167aB. C. Guo, A. W. Castleman, Jr., Chem. Phys. Lett. 1991, 181, 16;
- 167bD. Schröder, R. Wesendrup, R. H. Hertwig, T. K. Dargel, H. Grauel, W. Koch, B. R. Bender, H. Schwarz, Organometallics 2000, 19, 2608.
- 168Ref. [80c]
- 169Ref. [80b].
- 170T. S. Cameron, I. Dionne, H. D. B. Jenkins, S. Parsons, J. Passmore, H. K. Roobottom, Inorg. Chem. 2000, 39, 2042.
- 171W. V. Brooks, T. S. Cameron, S. Parsons, J. Passmore, M. Shriver, Inorg. Chem. 1994, 33, 6230.
- 172BE(O-O)=142 kJ mol−1 and BE(S-S)=267 kJ mol−1.
- 173However, the computation turned out to be very delicate and finally converged at the highly correlated CCSD(T)-cc-pV5Z level: H. D. B. Jenkins, L. C. Jitariu, I. Krossing, J. Passmore, R. Suontamo, J. Comput. Chem. 2000, 21, 218.
- 174Ref. [170].
- 175J. Powell, A. Lough, T. Saeed, J. Chem. Soc. Dalton Trans. 1997, 4137.
- 176X. Song, M. Thornton-Pett, M. Bochmann, Organometallics 1998, 17, 1004.
- 177W. V. Konze, B. L. Scott, G. J. Kubas, Chem. Commun. 1999, 1807.
- 178D. A. Walker, T. J. Woodman, D. L. Hughes, M. L. Bochmann, Organometallics 2001, 20, 3772.
- 179H. J. Frohn, S. Jakobs, G. Henkel, Angew. Chem. 1989, 101, 1534; Angew. Chem. Int. Ed. Engl. 1989, 28, 1506.
- 180I. Krossing, Dalton Trans. 2002, 500.
- 181To evaluate the FIA of SbnF2n (n=2, 3, 4) giving the fluoride-bridged ions [SbnF5n+1]− the FIA was calculated by two separate calculations: 1) The FIA of the doubly fluoride-bridged Al2F6 (D2h) for the formation of the singly fluoride-bridged [Al2F7]− ion was calculated based on the average of G2 and CBS-Q calculations as 501 kJ mol−1. This step is non-isodesmic but the G2 and CBS-Q levels are reported to reproduce experimental values with a uncertainty of less than 8 kJ mol−1 lending credibility to these values. 2) The FIA of SbnF2n (n=2, 3, 4) was then calculated by adding the reaction enthalpy of the isodesmic reaction [Al2F7]− + SbnF2n→[SbnF2n+1]− + Al2F6 to the FIA of Al2F6 (501 kJ mol−1; see Equations 37 a–d)
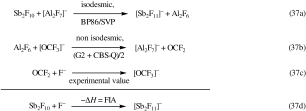 for an example for [Sb2F11]−). Similarly the FIA of [(RO)3Al-F-Al(OR)3]− and [F4C6{1,2-B(C6F5)2}2F]− was assessed in isodesmic reactions of [Al2F7]− and the Lewis acid 2 Al(OR)3 and F4C6{1,2-(B(C6F5)2}2 giving 2 AlF3 and the fluoride-bridged ion. From this reaction enthalpy and the FIA of 2 AlF3—determined as the average of G2 and CBS-Q calculations (−706 kJ mol−1)—the FIA of 2 Al(OR)3 and F4C6{1,2-(B(C6F5)2}2 was calculated (see Equation 38 a–d)
for an example for [Sb2F11]−). Similarly the FIA of [(RO)3Al-F-Al(OR)3]− and [F4C6{1,2-B(C6F5)2}2F]− was assessed in isodesmic reactions of [Al2F7]− and the Lewis acid 2 Al(OR)3 and F4C6{1,2-(B(C6F5)2}2 giving 2 AlF3 and the fluoride-bridged ion. From this reaction enthalpy and the FIA of 2 AlF3—determined as the average of G2 and CBS-Q calculations (−706 kJ mol−1)—the FIA of 2 Al(OR)3 and F4C6{1,2-(B(C6F5)2}2 was calculated (see Equation 38 a–d) for an example for [(RO)3Al-F-Al(OR)3]−).
for an example for [(RO)3Al-F-Al(OR)3]−).
- 182The LA was partitioned in two separate reactions: the first was an isodesmic reaction with which even very large systems could be calculated reliably at the BP86/SVP level [Eq. (39 a)
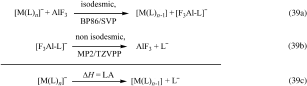 ]. The second reaction contains much smaller species, however, is non-isodesmic. Therefore the computationally much more time consuming but also more reliable MP2/TZVPP level was selected to assess the second part (It is currently impossible to run also the first reaction at the MP2 level). The LA was than obtained by a simple addition of both equations [Eq. (39 c)].
]. The second reaction contains much smaller species, however, is non-isodesmic. Therefore the computationally much more time consuming but also more reliable MP2/TZVPP level was selected to assess the second part (It is currently impossible to run also the first reaction at the MP2 level). The LA was than obtained by a simple addition of both equations [Eq. (39 c)].
- 183I. A. Koppel, P. Burk, I. Koppel, I. Leito, T. Sonoda, M. Mishima, J. Am. Chem. Soc. 2000, 122, 5114.
- 184I. Krossing, I. Raabe, Chem. Eur. J., submitted.
- 185R. J. Wehmschulte, J. M. Steele, J. D. Young, M. A. Khan, J. Am. Chem. Soc. 2003, 125, 1470.