Structure Determination in the Twilight Region Between Monolayers and 3-D Crystals; a Grazing Incidence X-Ray Diffraction Study of Nanocrystalline Aggregates of α,ω-Docosanediol at the Air–Water Interface†
Jaroslaw Majewski
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138
Search for more papers by this authorRon Edgar
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138
Search for more papers by this authorDr. Ronit Popovitz-Biro
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138
Search for more papers by this authorDr. Kristian Kjaer
Department of Physics Risø National Laboratory, DK-4000 Roskilde (Denmark)
Search for more papers by this authorDr. Wim G. Bouwman
Department of Physics Risø National Laboratory, DK-4000 Roskilde (Denmark)
Search for more papers by this authorProf. Jens Als-Nielsen
Department of Physics Risø National Laboratory, DK-4000 Roskilde (Denmark)
Search for more papers by this authorProf. Meir Lahav
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138
Search for more papers by this authorCorresponding Author
Professor Leslie Leiserowitz
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138Search for more papers by this authorJaroslaw Majewski
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138
Search for more papers by this authorRon Edgar
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138
Search for more papers by this authorDr. Ronit Popovitz-Biro
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138
Search for more papers by this authorDr. Kristian Kjaer
Department of Physics Risø National Laboratory, DK-4000 Roskilde (Denmark)
Search for more papers by this authorDr. Wim G. Bouwman
Department of Physics Risø National Laboratory, DK-4000 Roskilde (Denmark)
Search for more papers by this authorProf. Jens Als-Nielsen
Department of Physics Risø National Laboratory, DK-4000 Roskilde (Denmark)
Search for more papers by this authorProf. Meir Lahav
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138
Search for more papers by this authorCorresponding Author
Professor Leslie Leiserowitz
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138
Department of Materials and Interfaces The Weizmann Institute of Science, 76100 Rehovot (Israel) Telefax: Int. code + (8)34-4138Search for more papers by this authorWe acknowledge financial support from the Minerva Foundation, Munich, Germany.
Graphical Abstract
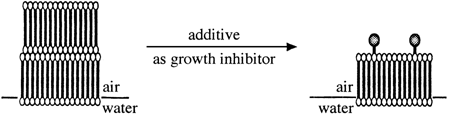
Double layers are formed by α,ω-docosanediol (C22 diol) when it is spread on a water surface. The space group (a slightly relaxed form of P21/a) and the lattice parameters of the unit cell were determined by X-ray diffraction. Furthermore, multilayer formation was inhibited by addition of 10% of C24H49OH or 5% of HO(CH2)22OCH2Ph to the spreading solution. The coverage with the double layer was reduced to less than 15%; the rest was occupied by a monolayer.
References
- 1 A bolaamphiphile contains a hydrophobic chain with hydrophilic groups at both ends. See G. H. Escamilla, G. R. Newkome, Angew. Chem. 1995, 106, 2016; Angew. Chem. Int. Ed. Engl. 1995, 33, 1937.
- 2 R. Popovitz-Biro, J. Majewski, L. Margulis, S. Cohen, L. Leiserowitz, M. Lahav, J. Phys. Chem. 1995, 98, 4970.
- 3 R. Popovitz-Biro, J. Majewski, L. Margulis, S. Cohen, L. Leiserowitz, M. Lahav, Adv. Mater. 1995, 6, 956.
- 4(a) S. Weinbach, K. Kjaer, W. G. Bouwman, G. Grübel, G. Legrand, J. Als-Nielsen, M. Lahav, L. Leiserowitz, Science 1995, 264, 1566; (b) S. Weinbach, K. Kjaer, J. Als-Nielsen, M. Lahav, L. Leiserowitz, J. Phys. Chem. 1993, 97, 5200.
- 5
J. Als-Nielsen,
K. Kjaer in
Proceedings of the NATO Advanced Study Institute Phase Transition in Soft Condensed Matter (Eds.:
T. Riste,
D. Sherrington),
Plenum, New York, Geilo, Norway,
1989, p. 113.
10.1007/978-1-4613-0551-4_11 Google Scholar
- 6 R. Popovitz-Biro, unpublished results.
- 7 The GID measurements were performed at the BW1 wiggler beamline at HASYLAB, DESY, Hamburg, Germany, on a liquid surface diffractometer. A beam of wavelength 1.35 Å was incident at a grazing angle slightly below the critical angle for total external reflection. This geometrical configuration enhances surface sensitivity. The footprint of the X-ray beam on the water surface was 5 × 50 mm. The backgound level of scattering was reduced by a He atmosphere inside the trough. Detection of both the horizontal (qxy) and vertical (qz) components of the diffracted beam was done by a vertical oriented positionsensitive detector (qz resolution) mounted behind a Soller collimator giving qxy resolution. The bolaamphiphiles were spread at room temperature from 5 × 10−4 M chloroform solution over the Millipore-filtered water contained in a Langmuir trough mounted on the diffractometer. GID measurements were performed after cooling of the subphase to 5 °C. More experimental details are given in Ref. [12].
- 8The GID pattern for 70,100, and 200% surface coverage were essentially the same, differing only in intensity and thus in the number of observable peaks.
- 9Two layers are found by GID in contrast to the two to five layers of the C22 diol observed after deposition on mica, by atomic force microscopy [2]. We may reconcile this difference by bearing in mind that the multilayers are prepared under dynamic conditions away from equilibrium, and so slight changes in temperature, pressure, and support may lead to structural differences.
- 10 D. M. Small in Handbook of Lipid Research, Vol. 4, Plenum, New York, 1986.
- 11 The b glide is ruled out since it would lead to a crisscrossed arrangement of chain axes and so to a poor packing, which has not yet been observed. Moreover, such a packing would not fit the Bragg rod profiles.
- 12 F. Leveiller, D. Jacquemain, L. Leiserowitz, K. Kjaer, J. Als-Nielsen, J. Phys. Chem. 1992, 96, 10380.
- 13 The inversion centers and twofold axes are crystallographically correct for the molecular chains, but only statistically correct for the hydroxyl hydrogen atoms in the hydrogen-bonding chain (Scheme 1 b), which must be disordered as in the 3D crystal structures of hexagonal ice [15a], methanol [15b], C16H33OH [15c], and C24H49OH [15d]. A completely ordered OH ··· O hydrogen-bonded system with identical OH ··· O repeat along the hydrogenbond chain axis a may be achieved with a twofold screw axis parallet to a. Such an arrangement would yield the commonly observed orthorhombic space group Pcab, but was found not to fit the GID data, which requires all the chains axes to be tilted in the same direction vis-a-vis the a axis.
- 14The best fit of the calculated Bragg rod intensity profiles to the observed data was obtained for an interlayer offset along the a axis of 3.3 Å and an interlayer separation of 30 Å. Furthermore, modeling with one or three layers yielded calculated Bragg rods with shapes distinctly different from those of the observed data.
- 15(a) D. W. Peterson, H. A. Levy, Acta Crystallogr. 1957, 10, 70; K. Shimaoko, J. Phys. Soc. Jpn. 1960, 15, 106; (b) K. J. Tauer, W. N. Lipscomb, Acta Crystallogr. 1952, 5, 606; (c) S. Abrahamsson, G. Larsson, E. von Sydow, Acta Crystallogr. 1960, 13, 770; (d) J.-L. Wang, F. Leveiller, D. Jacquemain, K. Kjaer, J. Als-Nielsen, M. Lahav, L. Leiserowitz, J. Am. Chem. Soc. 1995, 116, 1192.
- 16 D. Jacquemain, S. Grayer Wolf, F. Leveiller, K. Kjaer, M. Deutsh, J. Als Nielsen, M. Lahav, L. Leiserowitz, Angew. Chem. 1992, 104, 134; Angew. Chem. Int. Ed. Engl. 1992, 31, 130.
- 17Model structures made up of one or three layers did not fit the observed Bragg rod data satisfactorily. We also note that for the given grazing incidence geometry the intensity of the X-ray beam falls off exponentially with depth of penetration of a value I0/e (where I0 is the intensity of the incident beam) for the depth Λ ≈ 90 Å. If the thickness of the multilayer crystallites is less than 2 Λ, the shape of the Bragg rod is barely affected by the limited penetration depth. Furthermore the shape of the two-dimensional contour of the diffracted intensity I(qxy,qz) of the various reflections (not presented here) show that the ab plane of the multilayer crystallites are primarily parallel to the water surface. There is, however, some tendency for deviation therefrom. If such a spread of crystalline misorientation from the plane of water surface is pronounced, it would give rise to an apparent broadening of the Bragg rods along qz, which would have to be treated in a detailed analysis (K. Kjaer, W. G. Bouwman, unpublished work).
- 18For a chainlike hydrocarbon molecule the limited number of observable peaks in the GID data does not allow one to differentiate between two structures differing in orientation by 180° about the long molecular axis unless other criteria are introduced.
- 19This reduction in crystal symmetry was found to be necessary for a good fit to the GID data of monolayer structures of several amphiphilic molecules on water [12, 15d]. In the multilayer C22-diol structure the lean angles for the first and second layers were in opposite directions. In the refined structure the molecular chains are tilted by 1.5° along the a axis and leaned by 1.5° along the b axis.
- 20 In this packing the center of inversion and twofold axes relating the two layers in the two space group are lost. Moreover, the relaxation of the P21/a (or A2/a) symmetry is also manifested by the presence of an observed but very weak symmetry-forbidden {1,0} reflection.
- 21The results for symmetry relaxed from A2/a are very similar to that for P21/a and so are not shown here.
- 22 In addition to α and β phases a minor γ phase exhibiting two very weak Bragg rods was observed. Their intensities were approximately one hundredth those of the major phases. The qxy, qz positions of these two Bragg rods indicate a rectangular unit cell of dimensions a = 5.08 Å, b = 8.73 Å. Modeling yielded multilayer (trilayer) structure with the molecules tilted by about 30° from the vertical in the b direction.
- 23The intensity profiles of the {0,2} and {2,0} (not shown in Fig. 4a) reflections of monolayer phase α were used to derive a model of its structure. The calculated intensity contribution of this model structure was subtracted from the intensity profile of the unresolved {1,1} reflection. The remaining intensity profile together with the resolved {0,2} reflection of the β phase was well fitted to the calculated structure of the bilayer previously determined (Fig. 3). The unit cell of the monolayer was found to differ slightly from that of the double layer: a = 5.0, b = 7.43 Å.
- 24 A detailed determination of the multilayer structure would also benefit from atom-atom potential energy calculations as carried out on the amphiphilic monolayer structures [12].
- 25 The structures of the C16, C23, and C30 diols will be reported elsewhere.