New Selenolato-Bridged Clusters of Iron and Nickel; the Structures of [Fe12(SePh)24] and [Na2(POPh3)6][Ni20Se12(SeMe)10]†‡
Corresponding Author
Prof. Dr. Dieter Fenske
Institut für Anorganische Chemie der Universität, Engesserstrasse, Geb.-Nr. 30.45, D-76128 Karlsruhe (Germany) Telefax: Int. code + (721)661921
Institut für Anorganische Chemie der Universität, Engesserstrasse, Geb.-Nr. 30.45, D-76128 Karlsruhe (Germany) Telefax: Int. code + (721)661921Search for more papers by this authorDipl.-Chem. Andreas Fischer
Institut für Anorganische Chemie der Universität, Engesserstrasse, Geb.-Nr. 30.45, D-76128 Karlsruhe (Germany) Telefax: Int. code + (721)661921
Search for more papers by this authorCorresponding Author
Prof. Dr. Dieter Fenske
Institut für Anorganische Chemie der Universität, Engesserstrasse, Geb.-Nr. 30.45, D-76128 Karlsruhe (Germany) Telefax: Int. code + (721)661921
Institut für Anorganische Chemie der Universität, Engesserstrasse, Geb.-Nr. 30.45, D-76128 Karlsruhe (Germany) Telefax: Int. code + (721)661921Search for more papers by this authorDipl.-Chem. Andreas Fischer
Institut für Anorganische Chemie der Universität, Engesserstrasse, Geb.-Nr. 30.45, D-76128 Karlsruhe (Germany) Telefax: Int. code + (721)661921
Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 195) and the Fonds der Chemischen Industrie.
Dedicated to Professor Dr. Gerhard Fritz on the occasion of his 75th birthday
Graphical Abstract
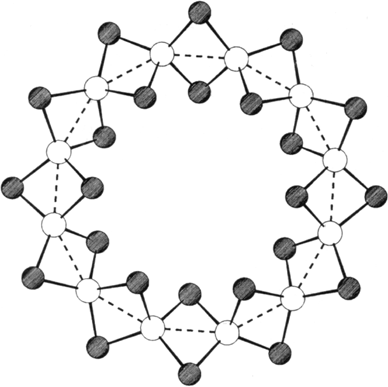
A twelve-membered ring made up of Fe2+ ions is present in the cluster framework of 1 (shown on the right without phenyl groups; Fe atoms are white, Se atoms shaded). The Fe2+ ions are located in the centers of edge-sharing Se4 tetrahedra, which in turn form a ring. The shiny black needles of 1 are prepared from FeCl3, PPh3, and PhSeSiMe3, and decompose readily upon heating.
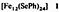
References
- 1
D. Fenske,
J. Ohmer,
J. Hachgenei,
K. Merzweiler,
Angew. Chem.
1988,
100, 1300;
Angew. Chem. Int. Ed. Engl.
1988,
27, 1277;
H. Krautscheid,
D. Fenske,
G. Baum,
M. Semmelmann,
Angew. Chem.
1993,
105, 1365;
10.1002/ange.19931050914 Google ScholarAngew. Chem. Int. Ed. Engl. 1993, 32, 1302; D. Fenske, H. Krautscheid, Angew. Chem. Int. Ed. Engl. 1990, 102, 1513 and Angew. Chem. Int. Ed. Engl. 1990, 29, 1453; D. Fenske, J. Steck, Angew. Chem. Int. Ed. Engl. 1993, 105, 254 and Angew. Chem. Int. Ed. Engl. 1993, 32, 238; S. Dehnen, A. Schäfer, D. Fenske, R. Ahlrichs, Angew. Chem. Int. Ed. Engl. 1994, 106, 786 and Angew. Chem. Int. Ed. Engl. 1994, 33, 746.
- 2 Crystal structure analyses: Stoe Stadi-IV and Stoe IPDS diffractometers (MoKα radiation, empirical absorption correction); data collection and refinement (SHELXS-86, SHELXL-93, SCHAKAL). 1·4C2H4CI2: lattice constants (200K): a = b = 2535.5(12), c = 1391.3(6) pm. α = β = γ = 90°, V = 8944.3 × 106 pm3; inversion twin space group P−421 c(no. 114), Z = 2. μ(MoKα) = 58.4 cm−1, 2θmax = 56, 25294 reflections, of which 10556 with I > 2σ( I), anisotropic refinement; R1 = 0.082. The phenyl groups are partially disordered. 1·5C2H4CI2: lattice constants (200K): a = 1372.3(9), b = 2542.4(12), c = 2578.3(12) pm, α = β = γ = 90°, V = 8995.5 × 106 pm3; space group Pmmn (no. 59), Z = 2, μ(MoKα) = 58.7 cm−1, 2 θmax = 52°, 9678 reflections, of which 5638 independent with I > 2σ( I), Fe, Se anisotropic, C isotropic, R1 = 0.086, R2 = 0.082. 2: lattice constants (200K): a = 1348.7(7), b = 1596.3(11), c = 1847.5(8) pm, α = 97.18(4), β = 106.71(14), γ = 103.05(3)°, V = 3633.5 x 106 pm3; space group P−1 (no. 2), Z = 1, μ(MoKα) = 79.5 cm−1, 2 θmax = 52°, 36312 reflections, of which 15851 independent with I > 4σ( I), 801 parameters (Ni, Se, P, O, Na, C anisotropic, the positions of the hydrogen atoms were calculated), R1 = 0.080, R2 = 0.076. Further details of the crystal structure investigation may be obtained from the Fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen (Germany) on quoting the depository number CSD-58694.
- 3 W. Bronger, P. Müller, J. Less Common Met. 1984, 100, 241; W. Bronger, Angew. Chem. 1981, 93, 12; Angew. Chem. Int. Ed. Engl. 1981, 20, 52; J. T. Hoggins, H. Steinfink, Acta Crystallogr. Sect. B 1977, 33, 673; J. T. Lemley, J. M. Jenks, J. T. Hoggins, Z. Eliezer, H. Steinfink, J. Solid State Chem. 1976, 16, 117; H. Y. Hong, H. Steinfink, J. Solid State Chem. 1972, 5, 93: J. S. Swinnea, H. Steinfink, J. Solid State Chem. 1980, 32, 329.
- 4 K. S. Hagen, R. H. Holm, Inorg. Chem. 1984, 23, 418.
- 5 K. S. Hagen, A. D. Watson, R. H. Holm, J. Am. Chem. Soc. 1983, 105, 3905.
- 6 J.-F. You, B. S. Snyder, R. H. Holm, J. Am. Chem. Soc. 1988, 110, 6589; J.-F. You, R. H. Holm, Inorg. Chem. 1991, 30, 1431; J.-F. You, G. C. Papaefthymiou, R. H. Holm, J. Am. Chem. Soc. 1992, 114, 2697.
- 7 J. Ensling, Universität Mainz, personal communication.
- 8 D. Gatteschi, A. Caneschi, L. Pardi, R. Sessoli, Science 1994, 265, 1054; K. L. Taft, S. Lippard, J. Am. Chem. Soc. 1990, 112, 9629.
- 9 J. J. Girerd, G. C. Papaefthymiou, A. D. Watson, E. Gamp, K. S. Hagen, N. Edelstein, R. B. Frankel, R. H. Holm, J. Am. Chem. Soc. 1984, 106, 5941; S. C. Lee, R. H. Holm, Angew. Chem. 1990, 102, 868; Angew. Chem. Int. Ed. Engl. 1990, 29, 840.
- 10 S. Otsuka, M. Kamata, K. Hirotsu, T. Higuchi, J. Am. Chem. Soc. 1981, 103, 3011; K. S. Hagen, G. Christou, R. H. Holm, Inrog. Chem. 1983, 22, 309; G. Christou, K. S. Hagen, J. K. Bashkin, R. H. Holm, Inrog. Chem. 1985, 24, 1010; M. Kriege, G. Henke, Z. Naturforsch. B. 1987, 42, 1121.
- 11 G. Henkel, M. Kriege, K. Matsumoto, J. Chem. Soc. Dalton Trans. 1988, 657.
- 12 D. Fenske, J. Ohmer, J. Hachgenei, Angew. Chem. 1985, 97, 993; Angew. Chem. Int. Ed. Engl. 1985, 24, 993.
- 13 T. Krüger, B. Krebs, G. Henkel, Angew. Chem. 1989, 101, 54; Angew. Chem. Int. Ed. Engl. 1989, 28, 61.
- 14 B. K. Teo, Inorg. Chem. 1984, 23, 1251; B. K. Teo, G. Longoni, F. R. K. Chung, Inorg. Chem. 1984, 23, 1257; B. K. Teo, J. Chem. Soc. Chem. Commun. 1983, 1362; D. M. P. Mingos, R. L. Johnston, Struct. Bonding (Berlin) 1987, 68, 29; D. M. P. Mingos, Chem. Soc. Rev. 1986, 15, 31; J. Chem. Soc. Chem. Commun. 1985, 1352.