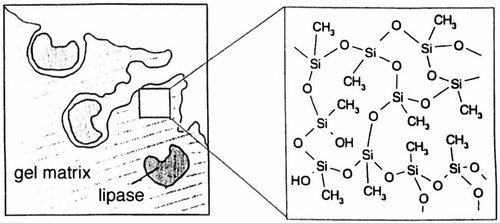
Efficient Heterogeneous Biocatalysts by Entrapment of Lipases in Hydrophobic Sol–Gel Materials
Corresponding Author
Prof. Dr. Manfred T. Reetz
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-45470 Mülheim an der Ruhr (Germany) Telefax: Int. code + (208)306-2985
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-45470 Mülheim an der Ruhr (Germany) Telefax: Int. code + (208)306-2985Search for more papers by this authorDipl.-Biochem. Albin Zonta
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-45470 Mülheim an der Ruhr (Germany) Telefax: Int. code + (208)306-2985
Search for more papers by this authorDr. Jörg Simpelkamp
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-45470 Mülheim an der Ruhr (Germany) Telefax: Int. code + (208)306-2985
Search for more papers by this authorCorresponding Author
Prof. Dr. Manfred T. Reetz
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-45470 Mülheim an der Ruhr (Germany) Telefax: Int. code + (208)306-2985
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-45470 Mülheim an der Ruhr (Germany) Telefax: Int. code + (208)306-2985Search for more papers by this authorDipl.-Biochem. Albin Zonta
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-45470 Mülheim an der Ruhr (Germany) Telefax: Int. code + (208)306-2985
Search for more papers by this authorDr. Jörg Simpelkamp
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-45470 Mülheim an der Ruhr (Germany) Telefax: Int. code + (208)306-2985
Search for more papers by this authorGraphical Abstract
Alkyltrimethoxysilanes yield much better host matrices for lipases than tetramethoxysilane (see picture). This is revealed by the esterifications discussed herein catalyzed by lipases that are immobilized in RSi(OMe)3 gels. These heterogeneous biocatalysts are readily reusable and should also be suitable for continuous processes.
References
- 1
K. Faber,
Biotransformations in Organic Chemistry, Springer, Berlin,
1992;
10.1007/978-3-642-97423-6 Google ScholarEnzymes as Catalysts in Organic Synthesis (Ed.: M. P. Schneider), Reidel, Dordrecht, 1986;10.1007/978-94-009-4686-6 Google ScholarW. Boland, C. Frössl, M. Lorenz, Synthesis 1991, 1049.
- 2 A. Zaks, A. M. Klibanov, Proc. Natl. Acad. Sci. USA 1985, 82, 3192; A. M. Klibanov, Acc. Chem. Res. 1990, 23, 114.
- 3 E. Guibe-Jampel, G. Rousseau, Tetrahedron Lett. 1990, 31, 112; F. X. Malcata, H. R. Reyes, H. S. Garcia, C. G. Hill. Jr., C. H. Amundson. J. Am. Oil Chem. Soc. 1990, 67, 890; M. Arroyo, J. M. Moreno, J. V. Sinisterra, J. Mol. Catal. 1993, 83, 261; see also R. V. Parthasarathy, C. R. Martin, Nature 1994, 369, 298.
- 4 J. A. Bosley, J. C. Clayton, Biotechnol. Bioeng. 1994, 43, 934; M. Norin, J. Boutelje, E. Holmberg, K. Hult, Appl. Microbiol. Biotechnol. 1988, 28, 527.
- 5 Y. Kimura, A. Tanaka, K. Sonomoto. T. Nihira, S. Fukui, Appl. Microbiol. Biotechnol. 1983, 17, 107.
- 6 “Immobilisierte Lipasen in hydrophoben Sol-Gel-Materialien”: M. T. Reetz, A. Zonta, J. Simpelkamp (Max-Planck-Institut für Kohlenforschung), DE-A 4408152.9, 1994.
- 7
F. H. Dickey,
J. Am. Chem. Soc.
1955,
59, 695;
P. Johnson,
T. L. Whateley,
J. Colloid Interface Sci.
1971, 557;
L. M. Ellerby,
C. R. Nishida,
F. Nishida,
S. A. Yamanaka,
B. Dunn,
J. Selverstone Valentine,
J. J. Zink,
Science
1992,
225, 1113;
Y. Tatsu,
K. Yamashita,
M. Yamaguchi,
S. Yamamura,
H. Yamamoto,
S. Yoshikawa,
Chem. Lett.
1992, 1615;
S. Braun,
S. Rappoport,
S. Shtelzer,
R. Zusman,
S. Druckmann,
D. Avnir,
M. Ottolenghi in
Biotechnology: Bridging Research and Application (Eds.:
D. Kamely,
A. Shakrabarty,
S. E. Kornguth),
Kluwer, Boston,
1991, p. 205;
10.1007/978-94-011-3456-9_14 Google ScholarH. Weetall, B. Robertson, D. Cullin, J. Brown, M. Walch, Biochem. Biophys. Acta 1993, 142, 211.
- 8 L. L. Hench, J. K. West, Chem. Rev. 1990, 90, 33; C. J. Brinker, W. Scherer, Sol-Gel-Science: The Physics and Chemistry of Sol-Gel-Processing, Academic Press, Boston, 1990.
- 9 A. M. Brzozowski, U. Derewenda, Z. S. Derewenda, G. G. Dodson, D. W. Lawson, J. P. Turkenburg, F. Bjorkling, B. Huge-Jensen, S. A. Patkar, L. Thim, Nature (London) 1991, 351, 491; K.-E. Jaeger, S. Ransac, B. W. Dijkstra, C. Colson, M. van Heuvel, O. Missef, FEMS Microbiol. Rev. 1994, 15, 29.
- 10 F. Schwertfeger, W. Glaubitt, U. Schubert, J. Non-Cryst. Solids 1992, 145, 85; Y. Haruvy, A. Heller, S. E. Weber in Supramolecular Architecture-Synthetic Control in Thin Films and Solids (Ed.: T. Bein) ( ACS Symp. Ser. 1992, 499), p. 405.
- 11 The lipase was dissolved in water or buffer, centrifuged to remove undissolved solids, and mixed with aqueous solutions of polyvinyl alcohol and sodium fluoride. The silanes were then added in the order of increasing reactivity. The reaction mixture was thoroughly mixed and shaken until gelation occurred (ca. 1 min). The gels thus obtained were allowed to stand in sealed vessels for 24 h, dried for 3 days at 37 °C, washed with water, acetone, and pentane, dried and ground into a powder.
- 12 We thank Novo Nordisk A/S (Denmark) for a sample of SP 523 (NOVO) and Amano Enzyme Europe Ltd. (England) for PS lipase.
- 13 Whereas the commercial enzyme powder combined with the water produced from the reaction and formed a viscous residue which was difficult to separate, the sol–gel immobilized lipase could be recycled without any difficulty.
- 14 We thank Dr. B. Tesche (Fritz Haber Institut, Berlin) for the scanning electron micrographs.
- 15 D. Bianchi, P. Cesti, E. Battistel, J. Org. Chem. 1988, 53, 5531.
- 16 According to Cesti et. al. [15] the reaction resulted in 95% ee. However, it is known that the enantioselectivity of enzyme-catalyzed reactions can vary for different batchesm of enzyme.
- 17 The influence of other parameters, for example the use of additives, the stoichiometry water/silane, the amount of catalyst, and the use of other silane monomers are presently under investigation in our group.