Atom Economy—A Challenge for Organic Synthesis: Homogeneous Catalysis Leads the Way
Corresponding Author
Prof. Barry M. Trost
Department of Chemistry, Stanford University, Stanford, CA 94305-5080 (USA) Telefax: Int. code + (415)725-0259
Department of Chemistry, Stanford University, Stanford, CA 94305-5080 (USA) Telefax: Int. code + (415)725-0259Search for more papers by this authorCorresponding Author
Prof. Barry M. Trost
Department of Chemistry, Stanford University, Stanford, CA 94305-5080 (USA) Telefax: Int. code + (415)725-0259
Department of Chemistry, Stanford University, Stanford, CA 94305-5080 (USA) Telefax: Int. code + (415)725-0259Search for more papers by this authorGraphical Abstract
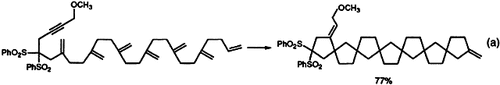
If all atoms of the starting materials are found in the product and only catalytic amounts of other reagents are needed, a reaction may be defined as ideal. A promising route to this ideal state is approached by the use of transition metal complexes as catalysts for addition and isomerization reactions. In this way, for instance, seven rings have been formed very efficiently from an acyclic precursor in a single step according to Equation (a).
Abstract
Enhancing the efficiency of the synthesis of complex organic products constitutes one of the most exciting challenges to the synthetic chemist. Increasing the catalogue of reactions that are simple additions or that minimize waste production is the necessary first step. Transition metal complexes, which can be tunable both electronically and sterically by varying the metal and/or ligands, are a focal point for such invention. Except for catalytic hydrogenation, such methods have been rare in complex synthesis and virtually unknown for CC bond formation until the advent of cross-coupling reactions. These complexes may orchestrate a variety of CC bond-forming processes, important for creation of the basic skeleton of the organic structure. Their ability to insert into CH bonds primes a number of different types of additions to relatively nonpolar π-electron systems. Besides imparting selectivity, they make feasible reactions that uncatalyzed were previously unknown. The ability of these complexes to preorganize π-electron systems serves as the basis both of simple additions usually accompanied by subsequent hydrogen shifts and of cycloadditions. The ability to generate “reactive” intermediates under mild conditions also provides prospects for new types of CC bond-forming reactions. While the examples reveal a diverse array of successes, the opportunities for new invention are vast and largely untapped.
References
- 1 B. M. Trost, Science 1991, 254, 1471.
- 2 I. Tkatchenko in Comprehensive Organometallic Chemistry, Vol. 8 (Ed.: G. Wilkinson), Pergamon, Oxford, 1982, p. 101; J. K. Stille in Comprehensive Organometallic Chemistry, Vol. 4 (Eds: B. M. Trost, I. Fleming, M. F. Semmelhack), Pergamon, Oxford, 1991, p. 913. For recent developments regarding selectivity, see G. D. Cuny, S. L. Buchwald, J. Am. Chem. Soc. 1993, 115, 2066; M. E. Broussard, B. Juma, S. G. Train, W.-J. Peng, S. A. Laneman, G. G. Stanley, Science 1993, 260, 1784; N. Sakai, S. Mano, K. Nozaki, H. Takaya, J. Am. Chem. Soc. 1993, 115, 7033.
- 3 J. Boor, Ziegler–Natta Catalysts and Polymerization, Academic Press, New York, 1979; Transition Metals and Organometallics as Catalysts for Olefin Polymerization (Eds.: W. Kaminsky, H. Sinn), Springer, Berlin, 1987; Transition Metal Catalyzed Polymerizations; Ziegler–Natta and Metathesis Polymerizations (Ed.: R. P. Quirk), Cambridge University Press, Cambridge, 1988; Catalytic Olefin Polymerization (Eds.: T. Keii, K. Soga), Elsevier, New York, 1990.
- 4 E. S. Brown in Organic Synthesis via Metal Carbonyls Vol. 2 (Eds.: I. Wender, P. Pino), Wiley-Interscience, New York, 1977, p. 655; C. A. Tolman, R. J. McKinney, W. C. Seidel, J. D. Druliner, W. R. Stevens, Adv. Catal. 1985, 33, 1.
- 5(a) Comprehensive Organic Synthesis Vol. 5 (Eds.: B. M. Trost, I. Fleming, L. A. Paquette), Pergamon, Oxford, 1991; (b) W. Oppolzer in ref. [5a], p. 315; (c) S. M. Weinreb in ref. [5a], p. 401; (d) D. L. Boger in ref. [5a], p. 451; (e) W. R. Roush in ref. [5a], p. 513; (f) U. Pindur, G. Lutz, C. Otto, Chem. Rev. 1993, 93, 741.
- 6 H. B. Kagan, D. Riant, Chem. Rev. 1992, 92, 1007.
- 7
S. G. Davies,
Organotransition Metal Chemistry. Applications to Organic Synthesis, Pergamon, Oxford, 1982, Chapter 7;
H. M. Colquhoun,
J. Holton,
D. J. Thompson,
M. V. Twigg,
New Pathways for Organic Synthesis, Plenum, New York, 1984, Chapter 5.
10.1007/978-1-4613-2651-9 Google Scholar
- 8 B. M. Trost, R. J. Kulawiec, J. Am. Chem. Soc. 1993, 115, 2027. For other Ru catalyzed isomerizations of allyl alcohols, see J. E. Bäckvall, U. Andreasson, Tetrahedron Lett. 1993, 34, 5459; D. V. McGrath, R. H. Grubbs, Organometallics 1994, 13, 224.
- 9 W. Ziegenbein in Chemistry of Acetylenes (Ed.: H. G. Viehe), Dekker, New York, 1969, pp. 169–256.
- 10 B. M. Trost, T. Schmidt, J. Am. Chem. Soc. 1988, 110, 2301.
- 11 C. Guo, X. Lu, J. Chem. Soc. Perkin Trans. 1 1993, 1921; X. Lu, J. Ji, D. Ma, W. Shen, J. Org. Chem. 1991, 56, 5774; D. Ma, X. Lu, Tetrahedron, 1990, 46, 3189, 6319; D. Ma, Y. Yu, X. Lu, J. Org. Chem. 1989, 54, 1105.
- 12Cf. H. Nemoto, H. N. Jimenez, Y. Yamamoto, J. Chem. Soc. Chem. Commun. 1990, 1304; H. Frauenrath, M. Sawicki, Tetrahedron Lett. 1990, 31, 649; D. P. Curran, P. B. Jacobs, R. L. Elliott, B. H. Kim, J. Am. Chem. Soc. 1987, 109, 5280.
- 13 S. Sato, H. Okada, I. Matsuda, Y. Izumi, Tetrahedron Lett. 1984, 25, 769.
- 14 K. Tani, T. Yamagata, S. Akutagawa, H. Kumobayashi, T. Taketomi, H. Takaya, A. Miyashita, R. Noyori, S. Otsuka, J. Am. Chem. Soc. 1984, 106, 5208.
- 15See also R. Schmid, J. Foricher, M. Cereghetti, P. Schönholzer, Helv. Chim. Acta 1991, 74, 370; R. Schmid, H. J. Hansen, Helv. Chim. Acta 1990, 73, 1258.
- 16 K. Takabe, Y. Uchiyama, K. Okisaka, T. Yamada, T. Katigeri, T. Okazaki, Y. Oketa, H. Kumobayashi, S. Akutagawa, Tetrahedron Lett. 1985, 26, 5153.
- 17 S. Akutagawa in Organic Synthesis in Japan: Past, Present and Future (Eds.: R. Noyori, T. Hiraoka, K. Mori, S. Murahashi, T. Onoda, K. Suzuki, O. Yonemitsu), Tokyo Kagaku Dodin, Tokyo, 1992, p. 75.
- 18 T. K. Hollis, W. Odenkirk, N. P. Robinson, J. Whelan, B. Bosnich, Tetrahedron (1993), 49, 5415. For a lanhanide catalyzed process, see S. Kobayashi, I. Hachiya J. Org. Chem. 1994, 59, 3590.
- 19 S. Sato, I. Matsuda, Y. Izumi, Chem. Lett. 1985, 1875. For nonmetal catalyzed reactions of this type, see H. M. R. Hoffmann, J. Rabe, J. Org. Chem. 1985, 50, 3849.
- 20 H. Sasai, T. Suzuki, S. Arai, T. Arai, M. Shibasaki, J. Am. Chem. Soc. 1992, 114, 4418.
- 21 H. Sasai, N. Itoh, T. Suzuki, M. Shibasaki, Tetrahedron Lett. 1993, 34, 855.
- 22 H. Nitta, D. Yu, M. Kudo, A. Mori, S. Inoue, J. Am. Chem. Soc. 1992, 114, 7969.
- 23 Y. Ito, M. Sawamura, T. Hayashi, J. Am. Chem. Soc. 1986, 108, 6405; T. Hayashi, M. Sawamura, Y. Ito, Tetrahedron 1992, 48, 1999.
- 24 A. Togni, S. D. Pastor, J. Org. Chem. 1990, 55, 1649; S. D. Pastor, A. Togni, Helv. Chim. Acta 1991, 74, 905.
- 25 T. Naota, H. Taki, M. Mizuno, S. I. Murahashi, J. Am. Chem. Soc. 1989, 111, 5954; S. Paganelli, A. Schionato, C. Botteghi, Tetrahedron Lett. 1991, 32, 2807.
- 26 M. Sawamura, H. Hamashima, Y. Ito, J. Am. Chem. Soc. 1992, 114, 8295; Tetrahedron 1994, 50, 4439. See also H. Brunner, B. Hammer, Angew. Chem. 1984, 96, 305; Angew. Chem. Int. Ed. Engl. 1984, 23, 312.
- 27 W. A. Nugent, R. J. McKinney, J. Org. Chem. 1985, 50, 5370.
- 28 T. V. RajanBabu, A. L. Casalnouvo, J. Am. Chem. Soc. 1992, 114, 6265.
- 29 J. E. Bäckvall, O. S. Andell, J. Chem. Soc. Chem. Commun. 1981, 1098.
- 30 W. R. Jackson, C. G. Lovel, J. Chem. Soc. Chem. Commun. 1982, 1231; G. D. Fallon, N. J. Fitzmaurice, W. R. Jackson, P. Perlmutter, J. Chem. Soc. Chem. Commun. 1985, 4; W. R. Jackson, P. Perlmutter, A. J. Smallridge, Tetrahedron Lett. 1988, 29, 1983.
- 31 O. S. Andell, J. E. Bäckvall, C. Moberg, Acta Chem. Scand. Ser. B 1986, 40, 184.
- 32 G. W. Parshall, S. D. Ittel, Homogeneous Catalysis, Wiley-Interscience, New York, 1992, pp. 42–45.
- 33 B. M. Trost, L. Zhi, Tetrahedron Lett. 1992, 33, 1831. See also P. W. Jolly, N. Kokel, Synthesis 1990, 771.
- 34 C. Mercier, G. Mignani, M. Aufrand, G. Allmang, Tetrahedron Lett. 1991, 32, 1433. See also G. Mignani, D. Morel, Y. Colleville, Tetrahedron Lett. 1985, 26, 6337.
- 35 B. M. Trost, G. A. Molander, J. Am. Chem. Soc. 1981, 103, 5969. See also J. Tsuji, H. Kataoka, Y. Kobayashi, Tetrahedron Lett. 1981, 22, 2575.
- 36 G. I. Nikishin, I. P. Kovalev, Tetrahedron Lett. 1990, 31, 7063.
- 37 T. Mitsudo, Y. Nakagawa, K. Watanabe, Y. Hori, H. Misawa, H. Watanabe, Y. Watanabe, J. Org. Chem. 1985, 50, 565.
- 38 B. M. Trost, C. Chan, C. Rühter, J. Am. Chem. Soc. 1987, 109, 3486.
- 39 B. M. Trost, J. J. Caringi, unpublished.
- 40 B. M. Trost, A. Harms, unpublished.
- 41 U. Schwieter, G. Saucy, M. Montavon, C. von Planta, R. Rüegg, O. Isler, Helv. Chim. Acta 1962, 45, 517; C. von Planta, U. Schwieter, L. Chopard-dit-Jean, R. Rüegg, M. Kofler, O. Isler, Helv. Chim. Acta 1962, 45, 548. For another synthesis and application, see W. R. Roush, B. B. Brown, J. Am. Chem. Soc. 1993, 115, 2268.
- 42 B. M. Trost, W. Brieden, K. H. Baringhaus, Angew. Chem. 1992, 104, 1392; Angew. Chem. Int. Ed. Engl. 1992, 31, 1335.
- 43 M. Akita, H. Yasuda, A. Nakamura, Bull. Chem. Soc. Jpn. 1984, 57, 480.
- 44 B. M. Trost, G. Kottirsch, J. Am. Chem. Soc. 1990, 112, 2816.
- 45 T. Kondo, M. Akazome, Y. Tsuji, Y. Watanabe, J. Org. Chem. 1990, 55, 1286. See also T. B. Marder, D. C. Roes, D. Milstein, Organometallics 1988, 7, 1451.
- 46 T. Tsuda, T. Kujor, T. Saegusa, J. Org. Chem. 1990, 55, 2554.
- 47 S. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda, N. Chatani, Nature (London) 1993, 336, 529.
- 48 P. W. Jolly, G. Wilke, The Organic Chemistry of Nickel, Vol. II, Academic Press, New York, 1975.
- 49
J. Tsuji,
Adv. Organometallics Chem.
1979,
17, 141;
J. Tsuji,
Organic Synthesis with Palladium Compounds, Springer, Berlin, 1980, pp. 90–125.
10.1007/978-3-642-67475-4 Google Scholar
- 50 R. Baker, A. H. Cook, T. N. Smith, J. Chem. Soc. Perkin Trans. 2 1974, 1517.
- 51 J. Tsuji, Bull. Chem. Soc. Jpn. 1973, 46, 1896.
- 52 D. Rose, H. Lepper, J. Organometallics Chem. 1973, 49, 473.
- 53 J. Tsuji, K. Mizutani, I. Shimizu, K. Yamamoto, Chem. Lett. 1976, 773.
- 54 J. Tsuji, T. Mandai, Tetrahedron Lett. 1978, 1817.
- 55 J. Tsuji, I. Shimizu, H. Suzuki, Y. Naito, J. Am. Chem. Soc. 1979, 101, 5070.
- 56 G. W. Parshall, S. D. Ittel, Homogeneous Catalysis, Wiley-Interscience, New York, 1992, pp. 126–127, 680.
- 57 W. A. Nugent, F. W. Hobbs, Jr., J. Org. Chem. 1983, 43, 5364; W. A. Nugent, R. J. McKinney, J. Mol. Catal. 1985, 29, 65.
- 58
B. Bogdanović,
B. Henc,
B. Meister,
H. Pauling,
G. Wilke,
Angew. Chem.
1972,
84, 1070;
10.1002/ange.19720842118 Google ScholarAngew. Chem. Int. Ed. Engl. 1972, 11, 1023.
- 59 G. Wilke, Angew. Chem. 1988, 100, 189; Angew. Chem. Int. Ed. Engl. 1988, 27, 185.
- 60 G. Buono, C. Siv, G. Peiffer, C. Triantaphylides, P. Denis, A. Mortreux, F. Petit, J. Org. Chem. 1985, 50, 1781. See also B. Bogdanović, B. Henc, A. Lösler, B. Meister, H. Pauling, G. Wilke, Angew. Chem. 1973, 85, 1013; Angew. Chem. Int. Ed. Engl. 1973, 12, 954.
- 61See, however, T. Mitsudo, S. Zhang, M. Nagao, Y. Watanabe, J. Chem. Soc. Chem. Commun. 1991, 598.
- 62 B. M. Trost, A. F. Indolese, J. Am. Chem. Soc. 1993, 115, 4361.
- 63 B. M. Trost, J. A. Martinez, R. J. Kulawiec, A. F. Indolese, J. Am. Chem. Soc. 1993, 115, 10402.
- 64 B. M. Trost, T. J. J. Müller, J. Am. Chem. Soc. 1994, 116, 4985.
- 65 M. M. Kabat, M. Lange, P. M. Wovkulich, M. R. Uskokovic, Tetrahedron Lett. 1992, 33, 7701; K. Mikami, M. Terada, T. Nakai, J. Am. Chem. Soc. 1990, 112, 3949.
- 66 T. Nakano, Y. Shimada, R. Sako, M. Kayama, H. Matsumoto, Y. Nagai, Chem. Lett. 1982, 1255.
- 67 Z. Yang, D. J. Burton, Tetrahedron Lett. 1991, 32, 1019. See also J. Tsuji, K. Sato, H. Nagashima, Tetrahedron 1985, 41, 393.
- 68 K. Kaneda, T. Uchiyama, Y. Fujiwara, T. Imanaka, S. Teranishi, J. Org. Chem. 1979, 44, 55.
- 69 B. M. Trost, G. Dyker, R. J. Kulawiec, J. Am. Chem. Soc. 1990, 112, 7809.
- 70 B. M. Trost, R. J. Kulawiec, J. Am. Chem. Soc. 1992, 114, 5579.
- 71 B. M. Trost, R. J. Kulawiec, A. Hammes, Tetrahedron Lett. 1993, 34, 587.
- 72 B. M. Trost, J. A. Flygare, J. Org. Chem. 1994, 59, 1078.
- 73 B. M. Trost, J. A. Flygare, J. Am. Chem. Soc. 1992, 114, 5476.
- 74 B. M. Trost, J. A. Flygare, Tetrahedron Lett. 1994, 35, 4059.
- 75 R. Stammler, M. Malacria, Synlett 1994, 92.
- 76 B. M. Trost, L. Zhi, unpublished.
- 77 B. M. Trost, R. W. Warner, J. Am. Chem. Soc. 1982, 104, 6112; J. Am. Chem. Soc. 1983, 105, 5940.
- 78 B. M. Trost, J. T. Hane, P. Metz, Tetrahedron Lett. 1986, 27, 5695.
- 79 A. S. Kende, I. Kaldor, R. Aslanian, J. Am. Chem. Soc. 1988, 110, 6265.
- 80 B. M. Trost, B. A. Vos, C. M. Brzezowski, D. P. Martina, Tetrahedron Lett. 1992, 33, 717.
- 81 B. M. Trost, S. Matsubara, J. J. Caringi, J. Am. Chem. Soc. 1989, 111, 8745.
- 82 B. M. Trost, S. Mallart, unpublished.
- 83 R. E. Campbell, C. F. Lochow, K. P. Vora, R. G. Miller, J. Am. Chem. Soc. 1980, 102, 5824; R. C. Larock, K. Oertle, G. F. Potter, J. Am. Chem. Soc. 1980, 102, 190.
- 84 K. P. Gable, G. A. Benz, Tetrahedron Lett. 1991, 32, 3473.
- 85 X. M. Wu, K. Funakoshi, K. Sakai, Tetrahedron Lett. 1993, 34, 5927. See also R. W. Barnhart, X. Wang, P. Noheda, S. H. Bergens, J. Whelan, B. Bosnich, Tetrahedron 1994, 50, 4335.
- 86(a) B. M. Trost, D. C. Lee, F. Rise, Tetrahedron Lett. 1989, 30, 651. (b) See also B. M. Trost, M. Lautens, C. Chan, D. J. Jebaratnam, T. Mueller, J. Am. Chem. Soc. 1991, 113, 636.
- 87 B. M. Trost, M. Lautens, Tetrahedron Lett. 1985, 26, 4887.
- 88 B. M. Trost, L. T. Phan, Tetrahedron Lett. 1993, 34, 4735.
- 89 B. M. Trost, A. S. Tasker, G. Rühter, A. Brandes, J. Am. Chem. Soc. 1991, 113, 671.
- 90 B. M. Trost, D. J. Jebaratnam, Tetrahedron Lett. 1987, 28, 1611. According to the work of M. Miyashita, T. Suzuki, A. Yoshikoshi, J. Am. Chem. Soc. 1989, 111, 3728, our synthesis also provides an access to picrotoxinin.
- 91 B. M. Trost, K. Matsuda, J. Am. Chem. Soc. 1988, 110, 5233.
- 92 B. M. Trost, J. M. Tour, J. Am. Chem. Soc. 1987, 109, 5268.
- 93 B. M. Trost, J. M. Tour, J. Am. Chem. Soc. 1988, 110, 5231.
- 94 B. M. Trost, G. J. Tanoury, M. Lautens, C. Chan, D. T. MacPherson, J. Am. Chem. Soc. 1994, 116, 4255.
- 95 B. M. Trost, D. L. Romero, F. Rise, J. Am. Chem. Soc. 1994, 116, 4268.
- 96 B. M. Trost, J. Y. L. Chung, J. Am. Chem. Soc. 1985, 107, 4586; B. M. Trost, P. A. Hipskind, J. Y. L. Chung, C. Chan, Angew. Chem. 1989, 101, 1559, Angew. Chem. Int. Ed. Engl. 1989, 28, 1502.
- 97 B. M. Trost, P. A. Hipskind, Tetrahedron Lett. 1992, 33, 4541.
- 98 B. M. Trost, D. C. Lee, J. Org. Chem. 1989, 54, 2271.
- 99 B. M. Trost, L. Zhi, K. Imi, Tetrahedron Lett. 1994, 35, 1361.
- 100 W. E. Piers, P. J. Shapiro, E. E. Bunel, J. E. Bercaw, Synlett 1990, 74.
- 101 R. Grigg, J. F. Malone, T. R. B. Mitchell, A. Ramasubbu, R. M. Scott, J. Chem. Soc. Perkin Trans. 1 1985, 1745.
- 102 B. Bogdanović, Adv. Organometallics Chem. 1979, 17, 105.
- 103 B. M. Trost, Y. Shi, J. Am. Chem. Soc. 1991, 113, 701; J. Am. Chem. Soc. 1993, 115, 9421.
- 104 B. M. Trost, Y. Shi, J. Am. Chem. Soc. 1992, 114, 791; J. Am. Chem. Soc. 1993, 115, 12491.
- 105 G. A. Molander, J. O. Hoberg, J. Am. Chem. Soc. 1992, 114, 3123.
- 106 B. M. Trost, F. Rise, J. Am. Chem. Soc. 1987, 109, 3161.
- 107 B. M. Trost, Y. Kondo, Tetrahedron Lett. 1991, 32, 1613; B. M. Trost, E. D. Edstrom, Angew. Chem. 1990, 102, 541; Angew. Chem. Int. Ed. Engl. 1990, 29, 520. See also B. M. Trost, E. D. Edstrom, M. B. Carter–Petillo, J. Org. Chem. 1989, 54, 4489.
- 108 B. M. Trost, G. J. Tanoury, J. Am. Chem. Soc. 1987, 109, 4753.
- 109 J. M. Takacs, L. G. Anderson, M. W. Creswell, B. E. Takacs, Tetrahedron Lett. 1987, 28, 5627. See also J. M. Takacs, P. W. Newsome, C. Kuehn, F. Takusagawa, Tetrahedron 1990, 46, 5507; J. M. Takacs, Y. C. Myoung, Tetrahedron Lett. 1992, 33, 317.
- 110 J. M. Takacs, S. V. Chandramouli, J. Org. Chem. 1993, 58, 7315.
- 111For an asymmetric Alder ene cyclization, see K. Narasaka, Y. Hayashi, S. Shimada, Chem. Lett. 1988, 1609.
- 112 K. Sakai, X. M. Wu, T. Hata, N. Marubayashi, K. Funakoshi, J. Chem. Soc. Perkin Trans. 1 1991, 2531; K. Funakoshi, N. Togo, Y. Taura, K. Sakai, Chem. Parm. Bull. 1989, 37, 1990.
- 113 B. M. Trost, J. F. Luengo, J. Am. Chem. Soc. 1988, 110, 8239.
- 114 H. Ishibashi, N. Uemura, H. Nakatani, M. Okazaki, T. Sato, N. Nakamura, M. Ikeda, J. Org. Chem. 1993, 58, 2360; G. M. Lee, S. M. Weinreb, J. Org. Chem. 1990, 55, 1281.
- 115 T. Yamamoto, S. Ishibuchi, T. Ishizuka, M. Haratake, T. Kunieda, J. Org. Chem. 1993, 58, 1997; F. O. H. Pirrung, H. Hiemstra, B. Kaptein, M. E. M. Sobrino, D. G. I. Petra, H. E. Shoemaker, W. N. Sperkamp, Synlett 1993, 739.
- 116 K. Narasaka, Y. Hayashi, H. Shimadzu, S. Niihata, J. Am. Chem. Soc. 1992, 114, 8869.
- 117 T. Mitsudo, H. Naruse, T. Kondo, Y. Ozaki, Y. Watanabe, Angew. Chem. 1994, 106, 595; Angew. Chem. Int. Ed. Engl. 1994, 33, 580.
- 118 H. tom Dieck, M. Mallien, R. Diercks, J. Mol. Catal. 1989, 51, 53.
- 119 A. J. Rippert, H. J. Hansen, Helv. Chim. Acta 1992, 75, 2219.
- 120 B. M. Trost, M. Yanai, K. Hoogsteen, J. Am. Chem. Soc. 1993, 115, 5294.
- 121 B. M. Trost, M. K. Trost, J. Am. Chem. Soc. 1991, 113, 1850. For a recent report of a Ru catalyzed reaction, see N. Chatani, T. Morimoto, T. Muto, S. Murai, J. Am. Chem. Soc. 1994, 116, 6049.
- 122 N. E. Schore in ref. [5a], Chapter 9.1 pp. 1037–1064.
- 123 V. Rautenstrauch, P. Mégard, J. Conesa, W. Küster, Angew. Chem. 1990, 102, 1441; Angew. Chem. Int. Ed. Engl. 1990, 29, 1413. See also N. Jeong, S. H. Hwang, Y. Lee, Y. K. Chung, J. Am. Chem. Soc. 1994, 116, 3159. For an iron catalyzed reaction of diallenes and allenyl carbonyl compounds, see B. Eaton, B. Rollman, J. A. Kaduk, J. Am. Chem. Soc. 1992, 114, 6245; M. S. Sigman, C. E. Kerr, B. E. Eaton, J. Am. Chem. Soc. 1993, 115, 7545.
- 124 S. C. Berk, R. B. Grossman, S. L. Buchwald, J. Am. Chem. Soc. 1993, 115, 4912.
- 125 K. Tamao, K. Kobayashi, Y. Ito, J. Am. Chem. Soc. 1988, 110, 1286.
- 126 P. Binger, H. M. Büch, Top. Curr. Chem. 1987, 135, 77. For intramolecular variants, see S. Yamago, E. Nakamura, Tetrahedron 1989, 45, 3081; R. T. Lewis, W. B. Motherwell, M. Shipman, J. Chem. Soc. Chem. Commun. 1988, 948.
- 127 B. M. Trost, T. Naota, unpublished.
- 128 E. P. Johnson, K. P. C. Vollhardt, J. Am. Chem. Soc. 1991, 113, 381.
- 129 M. Lautens, C. M. Crudden, Organometallics 1989, 8, 2733.
- 130 H. Brunner, M. Muschiol, F. Prester, Angew. Chem. 1990, 102, 680; Angew. Chem. Int. Ed. Engl. 1990, 29, 652; M. Lautens, J. C. Lautens, A. C. Smith, J. Am. Chem. Soc. 1990, 112, 5627.
- 131 M. Lautens, L. G. Edwards, Tetrahedron Lett. 1989, 30, 6813.
- 132 B. M. Trost, K. Imi, A. F. Indolese, J. Am. Chem. Soc. 1993, 115, 8831.
- 133 N. E. Schore, Chem. Rev. 1988, 88, 1081. For a recent leading reference, see J. E. Hill, G. Balaich, P. E. Fanwick, I. P. Rothwell, Organometallics 1993, 12, 2911.
- 134 P. Bhatarah, E. H. Smith, J. Chem. Soc. Chem. Commun. 1991, 277.
- 135 R. Grigg, R. Scott, P. Stevenson, Tetrahedron Lett. 1982, 23, 2691. See also S. J. Neeson, P. J. Stevenson, Tetrahedron Lett. 1988, 29, 813.
- 136 S. H. Lecker, N. H. Nguyen, K. P. C. Vollhardt, J. Am. Chem. Soc. 1986, 108, 857. See also Z. Zhou, L. P. Battaglia, G. P. Chiusoli, M. Costa, M. Nardelli, C. Pelizzi, G. Predieri, J. Chem. Soc. Chem. Commun. 1990, 1632.
- 137 G. Chelucci, M. Falorni, G. Giacomelli, Synthesis 1990, 1121.
- 138See also H. Bönnemann, W. Brijoux, Bull. Soc. Chim. Belg. 1985, 94, 635; R. E. Geiger, M. Lalonde, H. Stoller, K. Schleich, Helv. Chim. Acta 1984, 67, 1274; D. J. Brien, A. Naiman, K. P. C. Vollhardt, J. Chem. Soc. Chem. Commun. 1982, 133.
- 139 R. A. Earl, K. P. C. Vollhardt, J. Org. Chem. (1984), 49, 4786. For a review, see K. P. C. Vollhardt, Angew. Chem. 1984, 96, 525; Angew. Chem. Int. Ed. Engl. 1984, 23, 539.
- 140 A. C. Williams, P. Sheffels, D. Sheehan, T. Livinghouse, Organometallics 1989, 8, 1566.
- 141See A. J. Pearson, R. J. Shively, Jr., Organometallics 1994, 13, 578.
- 142 T. Tsuda, S. Morikawa, N. Hasegawa, T. Saegusa, J. Org. Chem. 1990, 55, 2978.
- 143 T. Tsuda, T. Kiyoi, T. Miyane, T. Saegusa, J. Am. Chem. Soc. 1988, 110, 8570.
- 144 I. Matsuda, M. Shibata, S. Sata, Y. Izumi, Tetrahedron Lett. 1987, 29, 3361.
- 145
H. tom Dieck,
R. Diercks,
Angew. Chem.
1984,
95, 801:
10.1002/ange.19830951014 Google ScholarAngew. Chem. Int. Ed. Engl. 1983, 22, 778.
- 146 R. S. Jolly, G. Luedtke, D. Sheehan, T. Livinghouse, J. Am. Chem. Soc. 1990, 112, 4965.
- 147 P. A. Wender, T. E. Jenkins, J. Am. Chem. Soc. 1989, 111, 6432.
- 148 T. E. Jenkins, Ph.D. Thesis, Stanford, University, 1992.
- 149 M. A. Huffman, L. S. Liebeskind, J. Am. Chem. Soc. 1991, 113, 2771.
- 150For an analogous transformation, see B. M. Trost, A. S. K. Hashmi, Angew. Chem. 1993, 105, 1130; Angew. Chem. Int. Ed. Engl. 1993, 32, 1085.
- 151 B. M. Trost, A. S. K. Hashmi, J. Am. Chem. Soc. 1994, 116, 2183.
- 152 K. Narasaka, N. Iwasawa, M. Inoue, T. Yamada, M. Nakashima, J. Sugimori, J. Am. Chem. Soc. 1989, 111, 5340.
- 153 S. Kobayashi, I. Hachiya, H. Ishitani, M. Araki, Tetrahedron Lett. 1993, 34, 4535.
- 154 K. Tamao, K. Kobayashi, Y. Ito, Synlett 1992, 539.
- 155 P. A. Wender, M. L. Snapper, Tetrahedron Lett. 1987, 28, 2221.
- 156 P. Brun, A. Tenaglia, B. Waegell, Tetrahedron Lett. 1983, 24, 385.
- 157 P. A. Wender, N. C. Ihle, C. R. D. Correia, J. Am. Chem. Soc. 1988, 110, 5904.
- 158 K.-U. Baldenius, H. tom Dieck, W. A. König, D. Icheln, T. Runge, Angew. Chem. 1992, 104, 338; Angew. Chem. Int. Ed. Engl. 1992, 31, 305.
- 159 J. H. Rigby, K. M. Short, H. S. Ateeq, J. A. Henshiwood, J. Org. Chem. 1992, 57, 5290.