Novel Alkynylcopper(I) Complexes and Lithium Alkynyl Cuprates†
Dr. Falk Olbrich
Institut für Anorganische und Angewandte Chemie der Universität, Martin-Luther-King-Platz 6, D-20146 Hamburg (FRG)
Search for more papers by this authorPriv. Doz. Dr. Jürgen Kopf
Institut für Anorganische und Angewandte Chemie der Universität, Martin-Luther-King-Platz 6, D-20146 Hamburg (FRG)
Search for more papers by this authorCorresponding Author
Prof. Dr. Erwin Weiss
Institut für Anorganische und Angewandte Chemie der Universität, Martin-Luther-King-Platz 6, D-20146 Hamburg (FRG)
Institut für Anorganische und Angewandte Chemie der Universität, Martin-Luther-King-Platz 6, D-20146 Hamburg (FRG)Search for more papers by this authorDr. Falk Olbrich
Institut für Anorganische und Angewandte Chemie der Universität, Martin-Luther-King-Platz 6, D-20146 Hamburg (FRG)
Search for more papers by this authorPriv. Doz. Dr. Jürgen Kopf
Institut für Anorganische und Angewandte Chemie der Universität, Martin-Luther-King-Platz 6, D-20146 Hamburg (FRG)
Search for more papers by this authorCorresponding Author
Prof. Dr. Erwin Weiss
Institut für Anorganische und Angewandte Chemie der Universität, Martin-Luther-King-Platz 6, D-20146 Hamburg (FRG)
Institut für Anorganische und Angewandte Chemie der Universität, Martin-Luther-King-Platz 6, D-20146 Hamburg (FRG)Search for more papers by this authorOn Metal–;Alkyl and Metal-Aryl Complexes, Part 48. This work was supported by the Fonds der Chemischen Industie. Part 47: S. Corbelin, N. P. Lorenzen, J. Kopf, E. Weiss, J. Organomet. Chem. 1991, 415, 293.
Graphical Abstract
References
- 1 Gmelin Handbook of Inorganic Chemistry, Cu, Organocupper Compounds, Part 3, 8th ed., Springer, Berlin, 1986.
- 2
J. G. Noltes,
G. van Koten in
Comprehensive Organometallic Chemistry,
Vol. 2
(Eds.: G. Wilkinson,
F. G. A. Stone,
E. W. Abel),
Pergamon, New York,
1982, p. 709 ff., and references therein.
10.1016/B978-008046518-0.00022-2 Google Scholar
- 3 N. P. Lorenzen, E. Weiss, Angew. Chem. 1990, 102, 322; Angew. Chem. Int. Ed. Engl. 1990, 29, 300.
- 4(a) P. W. R. Corfield, H. M. M. Shearer, Abstr. Am. Cryst. Assoc. Meeting, Bowman, MT USA, 1964, 96; (b) M. L. H. Green, Organometallic Compounds Vol. 2: The Transition Elements, 3rd ed., Methuen, London, 1968, p. 271.
- 5 F. Olbrich, U. Behrens, E. Weiss, unpublished.
- 6(a) A. E. Favorskii, L. Morev, J. Russ. Phys. Chem. Soc. 1924, 18, 2496; (b) H. O. House, M. J. Umen, J. Org. Chem. 1973, 38, 3893; (c) E. J. Corey, D. Floyd, B. H. Lipshutz, J. Org. Chem. 1978, 43, 3418.
- 7 H. O. House, C.-Y. Chu, J. M. Wilkins, M. J. Umen, J. Org. Chem. 1975, 40, 1460.
- 8 Crystal structure analysis of tetracosa[(tert-butylethynyl)copper(I)].2 n hexane (1): single crystals from n-hexane by slow cooling to 7°C and concentration of the solution. C144H216Cu24, dot; C12H28, triclinic, P1, Z = 1, a = 1419.1(2), b = 1526.3(2), c = 2228.4(5) pm, α = 104.80;(2), β = 91.03(2), γ = 117.32(2)°, V = 4094(2) × 106 pm3, ρcalcd = 1.478 gcm−3, μ = 35.1 cm−1 (absorption correction), crystal size 0.20 × 0.25 × 0.27 mm3, CuKα, Enraf Nonius CAD4 diffractometer, temperature 173 K, range 2.25° < θ < 76.5°, 13295 significant reflections (|F|) > 4σ(|F|), structure solution with direct methods (SHELXS, G. Sheldrick, Göttingen, 1986), 869 refined parameters, R = 0.047, Rw = 0.061 ([σ2 (F) + 0.0002 F2]−1, all non-hydrogen atoms refined with anisotropic temperature factors. In the asymmetric unit {(CuC-CtBu)12.n-hexane} the site of a tert-butylethynyl ligand (C90-C95) has 15% bromide occupancy (Br90). The hexane molecule is disordered. Drawing: E. Kelley, SCHAKAL 88, Freiburg, 1988).
- 9 Further details of the crystal structure investigation may be obtained from the Fachinformationszentrum Karlsruhe, Gesellschaft für wissenschaftlich-technische Information mbH, D-76344 Eggenstein-Leopoldshafen (FRG) on quoting the depository number CSD-56987, the names of the authors, and the journal citation.
- 10 M. Geissler, J. Kopf, B. Schubert, E. Weiss, W. Neugebauer, P. von R. Schleyer, Angew. Chem. 1987, 99, 569; Angew. Chem. Int. Ed. Engl. 1987, 26, 587.
- 11 N. P. Lorenzen, J. Kopf, F. Olbrich, U. Schümann, E. Weiss, Angew. Chem. 1990, 102, 1481; Angew. Chem. Int. Ed. Engl. 1990, 29, 1441.
- 12 M. I. Bruce, M. G. Humphrey, J. G. Matisons, S. K. Roy, A. G. Swincer, Aust. J. Chem. 1984, 37, 1955.
- 13 Crystal structure analysis of tetrakis[(μ3-tert-butylethynyl)triphenylphosphanecopper(I)].2 Et2O (2): single crystals from n-hexane. C96H96Cu4P4 C8H20O2, rhombohedral, P1, Z = 6, a = 1531.1(2), c = 6930(1) pm, V = 14069(3) × 106 pm3, ρcalcd = 1.257 gcm−3, μ = 13.8 cm−1, crystal size 0.43 × 0.28 × 0.18 mm3, MoKα, CAD4 diffractometer, temperature 293 K, range 2.25° < θ < 25.0°, 2377 significant reflections (|F|< 4σ (|F|)), direct methods, 402 refined parameters, R = 0.067, Rw, = 0.067 [σ2(F) + 0.0006 F2]−1, all non-hydrogen atoms refined with anisotropic temperature factors. The site of a tert-butylethynyl ligand (C20-C25) has 25% bromide occupancy (Br2). Both ether molecules are disordered; not all atoms could be localized.
- 14 L. Naldini, F. Demartin, M. Manassero, M. Sansoni, G. Rassu, M. A. Zoroddu, J. Organomet. Chem. 1985, 279, C42.
- 15 M. P. Gamasa, J. Gimeno, E. Lastra, X. Solans, J. Organomet. Chem. 1988, 346, 277.
- 16 B. H. Lipshutz, R. S. Wilhelm, J. A. Kozlowski (The Chemistry of Higher Order Organocuprates), Tetrahedron 1984, 40, 5005.
- 17 P. P. Power, (The Structures of Organocuprates and Heteroorganocuprates and Related Species in Solution and in the Solid State), Progr. Inorg. Chem. 1991, 39, 75.
- 18(a) S. H. Bertz, G. Dabbagh, J. Am. Chem. Soc. 1988, 110, 3668; (b) B. H. Lipshutz, S. Sharma, E. L. Ellsworth, J. Am. Chem. Soc. 1988, 110, 4032.
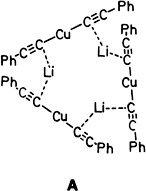
- 19 Crystal structure analysis of tris(diethyl ether)hexalithium deca(phenylethynyl)tetracuprate(i) (3) : single crystals from benzene. C92H80Cu4Li6O3, monoclinic, P21/c, Z = 4, a = 1763.6(3), b = 1938.3(3), c = 2308.4(3) pm, β = 91.34(1)°, V = 7889.2(2) × 106 pm3, ρcalcd = 1.288 gcm−3, μ = 15.4 cm−1, crystal size 0.30 × 0.20 × 0.05 mm3, CuKα, Enraf-Nonius CAD4 diffractometer, temperature 173 K, range 2.25° < θ < 60.0°, 7471 significant reflections (|F| < 3σ(|F|)), direct methods, 1105 refined parameters, R = 0.089, Rw = 0.090 ([σ2 (F)) + 0.001 F2]−1, all non-hydrogen atoms refined with anisotropic temperature factors.
- 20 U. Schümann, E. Weiss, Angew. Chem. 1988, 100, 573; Angew. Chem. Int. Ed. Engl. 1988, 27, 584.