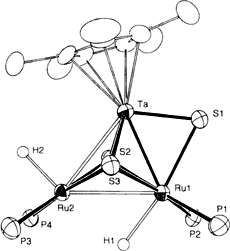
[CP*TaS3{Rh(cod)}2] and [CP*TaS3{RuH(PPh3)2}2]: A New Class of Heterometallic TaM2 Clusters
Corresponding Author
Prof. Dr. Kazuyuki Tatsumi
Department of Chemistry, Faculty of Engineering Science, Osaka University, Toyonaka, Osaka 560 (Japan), Telefax: Int. code +(6)857-3952
Kazuyuki Tatsumi, Department of Chemistry, Faculty of Engineering Science, Osaka University, Toyonaka, Osaka 560 (Japan), Telefax: Int. code +(6)857-3952
Roger E. Cramer, Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822 (USA)
Search for more papers by this authorHiroyuki Kawaguchi
Department of Chemistry, Faculty of Engineering Science, Osaka University, Toyonaka, Osaka 560 (Japan), Telefax: Int. code +(6)857-3952
Search for more papers by this authorYoshihisa Inoue
Department of Macromolecular Science, Faculty of Science, Osaka University, Toyonaka, Osaka 560 (Japan)
Search for more papers by this authorProf. Dr. Akira Nakamura
Department of Macromolecular Science, Faculty of Science, Osaka University, Toyonaka, Osaka 560 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Roger E. Cramer
Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822 (USA)
Kazuyuki Tatsumi, Department of Chemistry, Faculty of Engineering Science, Osaka University, Toyonaka, Osaka 560 (Japan), Telefax: Int. code +(6)857-3952
Roger E. Cramer, Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822 (USA)
Search for more papers by this authorProf. Dr. James A. Golen
Department of Chemistry, University of Massachusetts, Dartmouth, North Dartmouth, MA 02747 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Kazuyuki Tatsumi
Department of Chemistry, Faculty of Engineering Science, Osaka University, Toyonaka, Osaka 560 (Japan), Telefax: Int. code +(6)857-3952
Kazuyuki Tatsumi, Department of Chemistry, Faculty of Engineering Science, Osaka University, Toyonaka, Osaka 560 (Japan), Telefax: Int. code +(6)857-3952
Roger E. Cramer, Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822 (USA)
Search for more papers by this authorHiroyuki Kawaguchi
Department of Chemistry, Faculty of Engineering Science, Osaka University, Toyonaka, Osaka 560 (Japan), Telefax: Int. code +(6)857-3952
Search for more papers by this authorYoshihisa Inoue
Department of Macromolecular Science, Faculty of Science, Osaka University, Toyonaka, Osaka 560 (Japan)
Search for more papers by this authorProf. Dr. Akira Nakamura
Department of Macromolecular Science, Faculty of Science, Osaka University, Toyonaka, Osaka 560 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Roger E. Cramer
Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822 (USA)
Kazuyuki Tatsumi, Department of Chemistry, Faculty of Engineering Science, Osaka University, Toyonaka, Osaka 560 (Japan), Telefax: Int. code +(6)857-3952
Roger E. Cramer, Department of Chemistry, University of Hawaii at Manoa, Honolulu, HI 96822 (USA)
Search for more papers by this authorProf. Dr. James A. Golen
Department of Chemistry, University of Massachusetts, Dartmouth, North Dartmouth, MA 02747 (USA)
Search for more papers by this authorGraphical Abstract
Unsymmetric coordination of two Ru(H) (PPh3)2 complex fragments to the Cp*TaS3 unit is observed in the second title compound, which is depicted on the right without the Ph substituents. Three sulfido ligands link the Ru1 and two the Ru2 atom to the Ta atom. As a consequence the Ru1 – Ta distance is the shorter Ru–Ta bond.
References
- 1(a) K. Tatsumi, Y. Inoue, A. Nakamura, R. E. Cramer, W. VanDoorne, J. W. Gilje, J. Am. Chem. Soc. 1989, 111, 782; (b) K. Tatsumi, Y. Inoue, H. Kawaguchi, M. Kohasaka, A. Nakamura, R. E. Cramer, W. VanDoorne, G. J. Taogoshi, P. N. Richmann, Organometallics 1993, 12, 352.
- 2(a) E. Diemann, A. Müller, Coord. Chem. Rev. 1973, 10, 79; (b) A. Müller, S. Sarkar, Angew. Chem. 1977, 89, 748; Angew. Chem. Int. Ed. Engl. 1977, 16, 705; (c) A. Müller, H. Bögge, U. Schimansi, J. Chem. Soc. Chem. Commun. 1980, 91; (d) A. Müller, E. Krickemeyer, H. Bögge, Z. Anorg. Aug. Chem. 1987, 554, 61.
- 3(a) J. C. Huffman, R. S. Roth, A. R. Siedle, J. Am. Chem. Soc. 1976, 98, 4340; (b) J. K. Stalick, A. R. Siedle, A. D. Mighell, C. R. Hubbard, J. Am. Chem. Soc. 1979, 101, 2903; (c) J.-M. Manoli, C. Potvin, F. Sécheresse, J. Chem. Soc. Chem. Commun. 1982, 1159; (d) C. Potvin, J.-M. Manoli, F. Secheresse, S. Marzak, Inorg. Chem. 1987, 26, 4370; (e) P. Klingelhöfer, U. Müller, Z. Anorg. Aug. Chem. 1988, 556, 70.
- 4(a) D. Coucouvanis, Acc. Chem. Res. 1981, 14, 201, and references therein.; (b) D. Coucouvanis, E. D. Simhon, N. C. Baenzinger, J. Am. Chem. Soc. 1980, 102, 6644; (c) D. Coucouvanis, N. C. Baenzinger, E. D. Simhon, P. Stremple, D. Swenson, A. Kostikas, A. Simopoulos, V. Petrouleas, V. Papaefthymiou, J. Am. Chem. Soc. 1980, 102, 1732; (d) J. W. McDonald, G. D. Friesen, W. E. Newton, Inorg. Chim. Acta 1980, 46, L79.
- 5(a) K. E. Howard, T. B. Rauchfuss, A. L. Rheingold, J. Am. Chem. Soc. 1986, 108, 297; (b) K. E. Howard, T. B. Rauchfuss, S. R. Wilson, Inorg. Chem. 1988, 27, 3561; (c) K. E. Howard, J. R. Lockemeyer, M. A. Massa, T. B. Rauchfuss, S. R. Wilson, X. Yang, Inorg. Chem. 1990, 29, 4385.
- 6(a) Y. Do, E. D. Simhon, R. H. Holm, Inorg. Chem. 1985, 24, 4635; (b) Recently tetrathiometalates [MS4]3− of Nb and Ta were prepared and were found to give [MFe2S4Cl4] clusters. S. C. Lee, R. H. Holm, J. Am. Chem. Soc., 1990, 112, 9654.
- 7 Crystal structure analysis of 2 [10]: single crystal with dimensions 0.2 × 0.4 × 0.6 mm3, monoclinic space group C2/c, a = 33.29(1), b = 12.047(2), c = 13.711(3) Å, β = 90.50(2)°, V = 5498(2) Å3, Z = 8, ρcalcd = 2.016 Mg m−3. Data collection with a Nicolet R3m/V diffractometer; MoKα radiation, 2θ scans, 2θ = 4–60°, 8842 measured reflections, 8055 independent, 7469 observed (Fo > 1.0 σ Fo), empirical absorption correction based on azimuthal scans. Solution and refinement with the Nicolet SHELXTL PLUS package (direct methods); all non-hydrogen atoms were refined anisotropically and hydrogen atoms were put at calculated positions with a fixed isotropic U value; full matrix, refined parameters 283, R = 0.0347 and Rw = 0.0474, GOF = 0.96, maximum residual electron density 0.90 and −0.75 eÅ3.
- 8 The full width at half height (ν1/2) of the olefinic proton resonance at 30 °C is 8 Hz, and the coupling with the Rh nuclei was not detected. The corresponding resonance for [{Rh(cod)Cl}2] in C6D6 at room temperature comes at δ = 4.40 with ν1/2 = 10 Hz (100 MHz).
- 9 K. Tatsumi, H. Kawaguchi, K. Tani, R. E. Camer, J. W. Gilje, T. T. Bopp, unpublished.
- 10 Crystal structure analysis of 3: single crystal with dimensions 0.2 × 0.2 × 0.3 mm3, triclinic space group P−1, a = 12.84(1), b = 14.21(2), c = 21.620(9) Å, α = 81.5(1), β = 74.5(1), γ = 75.2(1)°, V = 3663(2) Å3, Z = 2, ρcalcd = 1.565 Mgm−3. Data collection with a Rigaku AFC5R diffractometer; MoKα radiation, ω-2θ scan, 2θ = 4–50.1°, 13 157 measured reflections, 13 098 independent, 9953 observed (I > 3.0 σI), empirical absorption correction based on azimuthal scans. Solution and refinement with the TEXSAN package of Molecular Structure Corporation (direct methods); all non-hydrogen atoms except for the THF solvent molecule were refined anisotropically and hydrogen atoms were put at calculated positions; full matrix, refined parameters 1064, R = 0.0331 and Rw = 0.0424, GOF = 1.63, maximum residual electron density 1.05 and −0.82 eÅ3. The molecule is disordered with major and minor occupancies of 0.9 and 0.1. In the minor configuration (see Fig. 2 bottom), S1 * bridges Ta* and Ru2 instead of Ta and Rut; the Ta*-Ru2 distance is 2.619(4) Å while the Ta*-Rul distance is longer at 3.206(5) Å. Due to its low occupancy, we were only able to locate two atoms, Ta* and S1*, of the minor configuration. This structure was solved twice with different crystals, once in Osaka and once in Honolulu. The Osaka results, which are slightly more accurate, are those reported here. Very similar disorder was found in both crystals. Further details of the structure analysis for 2 and 3 may be obtained from the Fachinformationszentrum Karlsruhe, Gesellschaft für wissenschaftlichtechnische Information mbH, D-W-7514 Eggenstein-Leopoldshafen 2 (FRG), on quoting the depository number CSD-57031, the names of the authors, and the journal citation.
- 11 The resonance of the hydrido ligand in [RuH(Cl)(PPh3)3] appears at δ = −17.75 with JPH = 26 Hz in CD2Cl2. P. S. Allman, B. R. McGarvey, G. Wilkinson, J. Chem. Soc. (A), 1968, 3143.