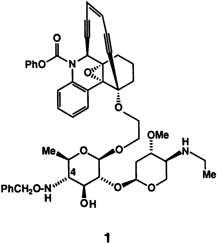
Synthetic Strategy for the Coupling of the Calicheamicin Oligosaccharide with Aglycons: Synthesis of Dynemicin A-Calicheamicin Hybrid Structures†
Corresponding Author
Prof. K. C. Nicolaou
Department of Chemistry Research Institute of Scripps Clinic 10666 N. Torrey Pines Road, La Jolla, California 92037 (USA) and Department of Chemistry University of California, San Diego la Jolla, California 92093 (USA)
Department of Chemistry Research Institute of Scripps Clinic 10666 N. Torrey Pines Road, La Jolla, California 92037 (USA) and Department of Chemistry University of California, San Diego La Jolla, California 92093 (USA)Search for more papers by this authorE. P. Schreiner
Department of Chemistry Research Institute of Scripps Clinic 10666 N. Torrey Pines Road, La Jolla, California 92037 (USA) and Department of Chemistry University of California, San Diego la Jolla, California 92093 (USA)
Search for more papers by this authorDr. W. Stahl
Department of Chemistry Research Institute of Scripps Clinic 10666 N. Torrey Pines Road, La Jolla, California 92037 (USA) and Department of Chemistry University of California, San Diego la Jolla, California 92093 (USA)
Search for more papers by this authorCorresponding Author
Prof. K. C. Nicolaou
Department of Chemistry Research Institute of Scripps Clinic 10666 N. Torrey Pines Road, La Jolla, California 92037 (USA) and Department of Chemistry University of California, San Diego la Jolla, California 92093 (USA)
Department of Chemistry Research Institute of Scripps Clinic 10666 N. Torrey Pines Road, La Jolla, California 92037 (USA) and Department of Chemistry University of California, San Diego La Jolla, California 92093 (USA)Search for more papers by this authorE. P. Schreiner
Department of Chemistry Research Institute of Scripps Clinic 10666 N. Torrey Pines Road, La Jolla, California 92037 (USA) and Department of Chemistry University of California, San Diego la Jolla, California 92093 (USA)
Search for more papers by this authorDr. W. Stahl
Department of Chemistry Research Institute of Scripps Clinic 10666 N. Torrey Pines Road, La Jolla, California 92037 (USA) and Department of Chemistry University of California, San Diego la Jolla, California 92093 (USA)
Search for more papers by this authorThis work was financially supported by the National Institutes of Health (USA) and the National Science Foundation (USA). W.S. is a Feoder Lynen Postdoctoral Fellow of the Alexander von Humboldt Foundation (1990–1991).
Graphical Abstract
An enediyne unit, an epoxide ring, and other functional groups characterize the aglycon moiety of 1. Compound 1 was synthesized stereoselectively by Schmidt coupling of the corresponding enediyne bridgehead alcohol after it had been converted into the hydroxyethyl compound by treatment with the trichloroacetimidate of the sugar moiety. The required reactions are tolerated by the highly sensitive functional groups in the molecule. This work brings the total synthesis of enediyne antibiotics one step closer.
References
- 1(a) J. Golik, J. Clardy, G. Dubay, G. Groenewold, H. Kawaguchi, M. Konishi, B. Krishnan, H. Ohkuma, K. Saitoh, T. W Doyle, J Am. Chem. Soc. 109 (1987) 3461; (b) J. Golik, G. Dubay, G. Groenewold, H. Kawaguchi, M. Konishi, B. Krishnan, H. Ohkuma, K. Saitoh, T. W. Doyle, J Am. Chem. Soc. 109 (1987) 3462.
- 2(a) M. D. Lee, T. S. Dunne, M. M. Siegel, C. C. Chang, G. O. Morton, D. B. Borders, J. Am. Chem. Soc 109 (1987) 3464; (b) M. D. Lee, T. S. Dunne, C. C. Chang, G. A. Ellestad, M. M. Siegel, G. O. Morton, W. J. McGahren, D. B. Borders, J. Am. Chem. Soc 109 (1987) 3466.
- 3 M. Konishi, H. Ohkuma, K. Matsumoto, T. Tsuno, H. Kamei, T. Miyaki, T. Oki, H. Kawagushi, G. D. VanDuyne, J. Clardy, J. Antibiot. 42 (1989) 1449; (b) M. Konishi, H. Ohkuma, T. Tsuno, T. Oki, G. D. VanDuyne, J. Clardy, J Am. Chem. Soc. 112 (1990) 3715.
- 4 K. C. Nicolaou, R. D. Groneberg, T. Miyazaki, N. A. Stylianides, T. J. Schultze, W. Stahl, J. Am. Chem. Soc 112 (1990) 8193; K. C. Nicolaou, R. D. Groneberg, J. Am. Chem. Soc 112 (1990) 4085.
- 5 K. C. Nicolaou, C.-K. Huang, A. L. Smith, S. V. Wendeborn, J. Am. Chem. Soc 112 (1990) 7416; K. C. Nicolaou, A. L. Smith, S. V. Wendeborn, C.-K. Huang, J. Am. Chem. Soc 113 (1991) in press. For another approach to a dynemicin A model, see: J. A. Porco, Jr., F. J. Schoenen, T. J. Stout, J. Clardy, S. L. Schreiber, J. Am. Chem. Soc 112 (1990) 7410.
- 6 T. Mukaiyama, Y. Muria, S. Shode, Chem. Lett. 1981, 431; K. C. Nicolaou, R. E. Dolle, D. P. Papahatjis, J. L. Randall, J. Am. Chem. Soc 106 (1984) 4159.
- 7 K. C. Nicolaou, R. D. Groneberg, N. A. Stylianides, T. Miyazaki, J. Chem. Soc. Chem. Commun. 1990, 1275.
- 8 S. David, A. Theiffry, J. Chem. Soc. Perkin Trans 1 1979, 1568.
- 9 U. Zenhavi, B. Amit, A. Patchornik, J. Org. Chem. 37 (1972) 2281; U. Zenhavi, A. Patchornik, J. Org. Chem. 37 (1972) 2285; E. Ohtsuka, S. Tanaka, M. Ikehara, J. Am. Chem. Soc. 100 (1978) 8210; V. N. R. Pillai, Synthesis 1980. 1.
- 10 G. Grandler, R. R. Schmidt, Carbohydr. Res. 135 (1985) 203; R. R. Schmidt, Angew. Chem. 98 (1986) 213; Angew. Chem. Int. Ed. Engl. 25 (1986) 212.
- 11 H. Paulsen, O. Lockhoff, Chem. Ber. 114 (1981) 3102.
- 12
All new compounds exhibited satisfactory spectral and analytical and/or exact mass data. Yields refer to spectroscopically and chromatographically homogenous materials. Selected physical properties for compounds 18, 26a and 26b (Ar = aryl). 18: pale yellow oil; Rf = 0.31 (silica, 10% methanol in dichloromethane), [α]
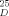 = −49.2 (c = 0.66, CHCl3); 1H NMR(500MHz,C6D6):δ=7.56(d,2H, J=7.6 Hz,Ar), 7.32–7.13(m. 8H, Ar),5.88(br. s, 1H, OH), 5.86(s, lH, E-1), 5.75 (br.s, 1H,O-NH), 4.99 (d, 1H, J = 11.7Hz, CH2Ph), 4.67 (d, lH, J = 11.7Hz. CH2Ph), 4.54–4.47 (m, 3H, A-1, CH2Ph-hydroxylamine), 4.32 (dd, 1 H J = 10.8, 9.2 Hz, E-5ax), 4.04 (dd, 1 H, J = 9.5, 9.5 Hz, A-3). 4.02–3.94 (m, 1 H, E-3),3.90(dd,1H. J = 10.8, 4.7 Hz, E-5eq), 3.88 (dd,lH, J=9.5,7.6Hz, A-2), 3.57(dq, 1H, J = 9.5, 6.0Hz, A-5), 3.23 (s,3H,OCH3),2.82(ddd, 1H, J= 9.2,9.2,4.7 Hz,E-4),2.57–2.42(m,3H, E-2eq,NCH2). 2.34(dd, 1 H, J= 9.5.9.5 Hz,A-4), 1.54(dd, 1 H, J= 10.2,10.2 Hz, E-2ax), 1.36(d, 3H, J = 6.0 Hz, A-6), 0.99 (t. 3H, J = 6.5 Hz, NCH2CH3); IR (CHCl3): ṽmax = 2964,2932, 1456,1095,1071 cm−1; HRMS calcd for C28H40N2O7, (M + Cs⊕) 649.1890; found 649.1900. 26a: pale yellow oil; Rf = 0.39 (silica, 10% methanol in dichloromethane), [α]
= −49.2 (c = 0.66, CHCl3); 1H NMR(500MHz,C6D6):δ=7.56(d,2H, J=7.6 Hz,Ar), 7.32–7.13(m. 8H, Ar),5.88(br. s, 1H, OH), 5.86(s, lH, E-1), 5.75 (br.s, 1H,O-NH), 4.99 (d, 1H, J = 11.7Hz, CH2Ph), 4.67 (d, lH, J = 11.7Hz. CH2Ph), 4.54–4.47 (m, 3H, A-1, CH2Ph-hydroxylamine), 4.32 (dd, 1 H J = 10.8, 9.2 Hz, E-5ax), 4.04 (dd, 1 H, J = 9.5, 9.5 Hz, A-3). 4.02–3.94 (m, 1 H, E-3),3.90(dd,1H. J = 10.8, 4.7 Hz, E-5eq), 3.88 (dd,lH, J=9.5,7.6Hz, A-2), 3.57(dq, 1H, J = 9.5, 6.0Hz, A-5), 3.23 (s,3H,OCH3),2.82(ddd, 1H, J= 9.2,9.2,4.7 Hz,E-4),2.57–2.42(m,3H, E-2eq,NCH2). 2.34(dd, 1 H, J= 9.5.9.5 Hz,A-4), 1.54(dd, 1 H, J= 10.2,10.2 Hz, E-2ax), 1.36(d, 3H, J = 6.0 Hz, A-6), 0.99 (t. 3H, J = 6.5 Hz, NCH2CH3); IR (CHCl3): ṽmax = 2964,2932, 1456,1095,1071 cm−1; HRMS calcd for C28H40N2O7, (M + Cs⊕) 649.1890; found 649.1900. 26a: pale yellow oil; Rf = 0.39 (silica, 10% methanol in dichloromethane), [α]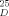 = −87.3 (c = 0.48, CHCl,), 1H NMR, (500 MHz, C6D6): δ = 8.97 (dd, 1 H, J = 4.2, 0.7 Hz, dyn-Ar), 7.52 (m. lH, dyn-Ar), 7.41–6.98 (m. 12H. 11 Ar, propargylic H), 6.98 (dd, 1 H, J =7.1, 7.1 Hz, dyn-Ar), 5.89 (bs, 1 H, OH), 5.83 (br. s, 1 H, 0-NH), 5.78 (s, 1 H, E-l), 5.28 (d, 1 H, J = 10.0 Hz, vinylic H), 5.10 (dd, 1 H, J = 10.0, 1.7 Hz, vinylic H), 4.58–4.51 (m, 2H, CH2Ph), 4.50 (d. lH, J=7.4,A-1),4.48–4.40(m,lH,E-5ax),4.25–4.17(m,lH,E-5eq) 4.13–4.02 (m, 3H, A-3,OCH2CH2O), 4.01–3.94 (m, 1H, OCH2CH2O), 3.93–3.90(m, 1H,E-3),3.77(dd, l H, J = 9.5, 7.3 Hz,A-2),3.69–3.52(m,lH,A-5),3.26(s, 3H,OCH3),2.78–2.66(m,2H,E-4,NCH2),2.65–2.57 (m,lH,NCH2),2.47(dd,1H, J= 12.0,2.2Hz,E-2eq),2.44(dd,lH, J= 9.5, 9.5 Hz, A-4). 2.31 (dd, 1 H, J = 14.5, 6.5 Hz, dyn-CH2), 2.04 (d, 1 H, J= 6.7 Hz, dyn-CH2), 1.95–1.83 (m, 4H, CH2), 1.43 (dd, lH, J = 14.5,9.3Hz. E-2ax), 1.33(d, 3H, J=6.1Hz,A-6), 1.04(t, 3H, J=6.5Hz, NCH2CH3); IR (CHCl3)ṽmax = 2965, 2931, 1733, 1380, 1323, 1146, 1098, 1071 cm−1; HRMS caled for C49H55N3O11 (M + Cs⊕) 994.2891; found 994.2904. 26b: pale yellow oil; Rf = 0.38 (silica, 10% methanol in dichloromethane), [α]
= −87.3 (c = 0.48, CHCl,), 1H NMR, (500 MHz, C6D6): δ = 8.97 (dd, 1 H, J = 4.2, 0.7 Hz, dyn-Ar), 7.52 (m. lH, dyn-Ar), 7.41–6.98 (m. 12H. 11 Ar, propargylic H), 6.98 (dd, 1 H, J =7.1, 7.1 Hz, dyn-Ar), 5.89 (bs, 1 H, OH), 5.83 (br. s, 1 H, 0-NH), 5.78 (s, 1 H, E-l), 5.28 (d, 1 H, J = 10.0 Hz, vinylic H), 5.10 (dd, 1 H, J = 10.0, 1.7 Hz, vinylic H), 4.58–4.51 (m, 2H, CH2Ph), 4.50 (d. lH, J=7.4,A-1),4.48–4.40(m,lH,E-5ax),4.25–4.17(m,lH,E-5eq) 4.13–4.02 (m, 3H, A-3,OCH2CH2O), 4.01–3.94 (m, 1H, OCH2CH2O), 3.93–3.90(m, 1H,E-3),3.77(dd, l H, J = 9.5, 7.3 Hz,A-2),3.69–3.52(m,lH,A-5),3.26(s, 3H,OCH3),2.78–2.66(m,2H,E-4,NCH2),2.65–2.57 (m,lH,NCH2),2.47(dd,1H, J= 12.0,2.2Hz,E-2eq),2.44(dd,lH, J= 9.5, 9.5 Hz, A-4). 2.31 (dd, 1 H, J = 14.5, 6.5 Hz, dyn-CH2), 2.04 (d, 1 H, J= 6.7 Hz, dyn-CH2), 1.95–1.83 (m, 4H, CH2), 1.43 (dd, lH, J = 14.5,9.3Hz. E-2ax), 1.33(d, 3H, J=6.1Hz,A-6), 1.04(t, 3H, J=6.5Hz, NCH2CH3); IR (CHCl3)ṽmax = 2965, 2931, 1733, 1380, 1323, 1146, 1098, 1071 cm−1; HRMS caled for C49H55N3O11 (M + Cs⊕) 994.2891; found 994.2904. 26b: pale yellow oil; Rf = 0.38 (silica, 10% methanol in dichloromethane), [α] = + 125.7 (c = 0.68, CHCl3), 1H NMR (500 MHz, C6D6): δ = 8.93 (dd, 1 H, J = 4.2, 0.7 Hz, dyn-Ar), 7.56 (dd, J =7.4, 1.4 Hz, dyn-Ar), 7.32–7.02 (m, 12H, 11 Ar, propargylic H), 6.90 (dd, 1 H, J = 7.1, 7.1 Hz, dyn-Ar), 5.90 (br. s, 1 H, 0-NH), 5.88 (br. s, 1 H, OH), 5.82 (s,lH,E-1), 5.29(d,lH, J= 10. 2Hz, vinylicH), 5.11 (dd,lH, J = 10.2, 1.7 Hz, vinylic H), 4.56–4.51 (m, 2HCH2Ph), 4.49 (dd, 1 H, J = 11.1,9.0Hz,E-5ax), 4.48 (d,lH, J =7.4Hz,A-l), 4.22–4.17(m,2H, OCH2CH2O), 4.14 (dd, 1 H, J = 11.1, 4.7 Hz, E-5eq), 4.09 (dd, 1 H, J=9.5, 9.5Hz, A-3), 4.06–4.01 (m, 1H, OCH2CH2O), 3.93–3.88 (m, lH, OCH2CH2O),3.87–3.81 (m,lH,E-3),3.78(dd,lH, J=9.5,7.1 Hz, lH, J =9.0, 9.0, 4.7Hz, E-4), 2.77 (m, 2H N-CH2), 2.54 (dd, lH, J = 12.2,2.5 Hz, E-2eq), 2.42(dd, 1 H, J = 9.5,9.5 Hz, A-4), 2.30 (dd, 1 H, J = 14.6, 10.5 Hz, dyn-CH), 2.06 (dd, 1 H, J = 14.6, 7.1 Hz, dyn-CH2), 1.97–1.83(m,4H,dyn- CH2), 1.51(dd,1H, J=12.2,9.2 Hz,E-2ax),1.33 (d, 3H, J = 6.1 HZ, A-6), 1.10 (t, 3H, J = 6.5 Hz, NCH2CH3); IR (CHCl3):ṽmax = 2962, 2957, 2929, 1733, 1386, 1323, 1146, 1097, 1070 cm−1; HRMS calcd for C49H55N3O11 (M + Cs⊕) 994.2891: found 994.2904.
= + 125.7 (c = 0.68, CHCl3), 1H NMR (500 MHz, C6D6): δ = 8.93 (dd, 1 H, J = 4.2, 0.7 Hz, dyn-Ar), 7.56 (dd, J =7.4, 1.4 Hz, dyn-Ar), 7.32–7.02 (m, 12H, 11 Ar, propargylic H), 6.90 (dd, 1 H, J = 7.1, 7.1 Hz, dyn-Ar), 5.90 (br. s, 1 H, 0-NH), 5.88 (br. s, 1 H, OH), 5.82 (s,lH,E-1), 5.29(d,lH, J= 10. 2Hz, vinylicH), 5.11 (dd,lH, J = 10.2, 1.7 Hz, vinylic H), 4.56–4.51 (m, 2HCH2Ph), 4.49 (dd, 1 H, J = 11.1,9.0Hz,E-5ax), 4.48 (d,lH, J =7.4Hz,A-l), 4.22–4.17(m,2H, OCH2CH2O), 4.14 (dd, 1 H, J = 11.1, 4.7 Hz, E-5eq), 4.09 (dd, 1 H, J=9.5, 9.5Hz, A-3), 4.06–4.01 (m, 1H, OCH2CH2O), 3.93–3.88 (m, lH, OCH2CH2O),3.87–3.81 (m,lH,E-3),3.78(dd,lH, J=9.5,7.1 Hz, lH, J =9.0, 9.0, 4.7Hz, E-4), 2.77 (m, 2H N-CH2), 2.54 (dd, lH, J = 12.2,2.5 Hz, E-2eq), 2.42(dd, 1 H, J = 9.5,9.5 Hz, A-4), 2.30 (dd, 1 H, J = 14.6, 10.5 Hz, dyn-CH), 2.06 (dd, 1 H, J = 14.6, 7.1 Hz, dyn-CH2), 1.97–1.83(m,4H,dyn- CH2), 1.51(dd,1H, J=12.2,9.2 Hz,E-2ax),1.33 (d, 3H, J = 6.1 HZ, A-6), 1.10 (t, 3H, J = 6.5 Hz, NCH2CH3); IR (CHCl3):ṽmax = 2962, 2957, 2929, 1733, 1386, 1323, 1146, 1097, 1070 cm−1; HRMS calcd for C49H55N3O11 (M + Cs⊕) 994.2891: found 994.2904.