Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter†
Corresponding Author
Dr. Donald A. Tomalia
Applied Organic and Functional Polymers Research, The Dow Chemical Company, Midland, MI 48674 (USA)
Michigan Molecular Institute 1910 W. St. Andrews Road, Midland. MI 48640 (USA)Search for more papers by this authorDr. Adel M. Naylor
Arthur Amos Noyes Laboratory of Chemical Physics, California Institute of Technology Pasadena, CA 91125 (USA)
Search for more papers by this authorProf. William A. Goddard III
Arthur Amos Noyes Laboratory of Chemical Physics, California Institute of Technology Pasadena, CA 91125 (USA)
Search for more papers by this authorCorresponding Author
Dr. Donald A. Tomalia
Applied Organic and Functional Polymers Research, The Dow Chemical Company, Midland, MI 48674 (USA)
Michigan Molecular Institute 1910 W. St. Andrews Road, Midland. MI 48640 (USA)Search for more papers by this authorDr. Adel M. Naylor
Arthur Amos Noyes Laboratory of Chemical Physics, California Institute of Technology Pasadena, CA 91125 (USA)
Search for more papers by this authorProf. William A. Goddard III
Arthur Amos Noyes Laboratory of Chemical Physics, California Institute of Technology Pasadena, CA 91125 (USA)
Search for more papers by this authorSTARBURST is a registered trademark of The Dow Chemical Company.
Graphical Abstract
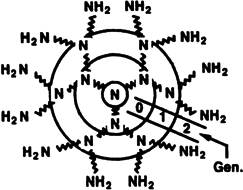
The synthesis of starburst polyamidoamines can be initiated by the addition of ammonia to methyl acrylate in a 1:3 ratio. The resulting triester is then treated with ethylenediamine in large excess, thereby affording a dendrimer of generation 0 containing three terminal NH2 groups. Reiteration of the two reactions leads 10 higher generations (generation 2 is shown here). Ideal growth results in dendrimers having a defined number of surface groups, which, in turn, may be modified chemically.
Abstract
Starburst dendrimers are three-dimensional, highly ordered oligomeric and polymeric compounds formed by reiterative reaction sequences starting from smaller molecules—“initiator cores” such as ammonia or pentaerythritol. Protecting group strategies are crucial in these syntheses, which proceed via discrete “Aufbau” stages referred to as generations. Critical molecular design parameters (CMDPs) such as size, shape, and surface chemistry may be controlled by the reactions and synthetic building blocks used. Starburst dendrimers can mimic certain properties of micelles and liposomes and even those of biomolecules and the still more complicated, but highly organized, building blocks of biological systems. Numerous applications of these compounds are conceivable, particularly in mimicking the functions of large biomolecules as drug carriers and immunogens. This new branch of “supramolecular chemistry” should spark new developments in both organic and macromolecular chemistry.
References
- 1 J. Swift: Gulliver's Travels (1726). Nal Penguin Inc., New York, Gullivers Reisen (Insel Taschenbuch 58), Insel Verlag, Frankfurt am Main 1974.
- 2(a) R. J. Leatherbarrow, A. R. Fersht, Protein Eng. 1 (1986) 7; (b) J. R. Knowles, Science (Washington) 236 (1987) 1252.
- 3(a) G. Nimtz, P. Marquardt, D. Stauffer, W. Weiss, Science (Washington) 242 (1988) 1671; (b) Z. Cai, C. R. Martin, J. Am. Chem. Soc. 111 (1989) 4138; (c) R. N. Barnett, U. Landman, D. Scharf, J. Jortner, Acc. Chem. Res. 22 (1989) 350; (d) J. Koutecky, P. Fantucci, Chem. Rev. 86 (1986) 539.
- 4(a)
E. T. Kaiser,
Angew. Chem.
100
(1989) 945;
10.1002/ange.19881000708 Google ScholarAngew. Chem. Int. Ed. Engl. 27 (1988) 913; E. T. Kaiser, H. Mihara, G. A. Laforet, J. W. Kelly, L. Walters, M. A. Findeis, T. Sassaki, Science (Washington) 243 (1989) 187; (b) M. Mutter, Angew. Chem. 97 (1985) 639; Angew. Chem. Int. Ed. Engl. 24 (1985) 639; M. Mutter, S. Vuilleumier, Angew. Chem. Int. Ed. Engl. 101 (1989) 551 and Angew. Chem. Int. Ed. Engl. 28 (1989) 535.
- 5(a)
H. Kuhn,
J. Waser,
Angew. Chem.
93
(1981) 435:
10.1002/ange.19810930604 Google ScholarAngew. Chem. Int. Ed. Engl. 20 (1981) 500; (b) H. Haken: Erfolgsgeheimnisse der Natur – Synergetik: Die Lehre vom Zusammenwirken, Deutsche Verlagsanstalt, Stuttgart 1986; (c) B. O. Küppers (Ed.): Ordnung aus dem Chaos. Prinzipien der Selbstorganisation und Evolution des Lebens, Piper. München 1987: (d) F. Cramer, Chaos und Ordnung. Die komplexe Strukture des Lebendigen, Deutsche Verlagsanstalt, Stuttgart 1988.
- 6(a) M. Eigen, Naturwissensehaften 10 (1971) 465; (b) M. Eigen, W. Gardiner, P. Schuster, R. Winkler-Oswatitch in J. M. Smith (Ed.): Evalutation Now, Freeman, New York, 1982, p. 11; (c) I. Prigogine, Phys. Today 25 (1972) No. 12, p. 38.
- 7 C. B. Anfinsen, Science (Washington) 181 (1973) 223; R. Jaenicke, Angew. Chem. 96 (1984) 285; Angew. Chem. Int. Ed. Engl. 96 (1984) 395.
- 8
C. J. Pedersen,
J. Am. Chem. Soc.
89
(1967) 7017;
C. J. Pedersen,
H. K. Frensdorff,
Angew. Chem.
84
(1972) 16;
Angew. Chem. Int. Ed. Engl.
11
(1972) 16;
C. J. Pedersen,
Angew. Chem. Int. Ed. Engl.
100
(1989)
1053 and
10.1002/ange.19881000805 Google ScholarAngew. Chem. Int. Ed. Engl. 27 (1988) 1029.
- 9(a) J. M. Lehn, Struct. Bonding (Berlin) 16 (1973) 1; Acc. Chem. Rev. 11 (1978) 49; (b) P. G. Potvin, J. M. Lehn in R. M. Izatt, J. J. Christensen (Eds): Synthesis of Macrocycles: The Design of Selective Complexing Agents (Prog. Macrocyclic Chem. 3, Wiley, New York 1987, p. 167; (c) J. M. Lehn, Angew. Chem. 100 (1988) 91; Angew. Chem. Int. Ed. Engl. 27 (1988) 89.
- 10
F. Vögtle,
E. Weber,
Angew. Chem.
91
(1970) 813;
10.1002/ange.19790911007 Google ScholarAngew. Chem. Int. Ed. Engl. 15 (1979) 753.
- 11 J. Rebek, Jr., Acc. Chem. Res. 17 (1984) 258; Science (Washington) 235 (1987) 1478; Angew. Chem. 102 (1990) No. 3; Angew. Chem. Int. Ed. Engl. 29 (1990) No. 3.
- 12(a) D. J. Cram, J. M. Cram, Acc. Chem. Res. 11 (1978) 8; (b) D. J. Cram, Angew. Chem. 98 (1986) 1041; Angew. Chem. Int. Ed. Engl. 27 (1986) 1039; (c) Angew. Chem. Int. Ed. Engl. 100 (1988) 104 and 27 Angew. Chem. Int. Ed. Engl. 100 (1988) 109 Angew. Chem. Int. Ed. Engl. 100 Science (Washington) 240 (1988) 760.
- 13(a) I. Tabushi, Acc. Chem. Res. 15 (1982) 66; (b) C. D. Gutsche, Acc. Chem. Res. 16 (1983) 161; (c) F. Diederich, Angew. Chem. 100 (1988) 372; Angew. Chem. Int. Ed. Engl. 27 (1988) 362.
- 14(a) F. A. Jurnak, A. McPherson (Eds.): Biological Macromolecules and Assemblies, Wiley, New York 1987; (b) J. Kraut, Science (Washington) 242 (1988) 533; (c) G. M. Whitesides, C.-H. Wong, Angew. Chem. 97 (1985) 617; Angew. Chem. Int. Ed. Engl. 24 (1985) 617.
- 15 R. F. Doolittle, Sci. Am. 253 (1985) No. 10, p. 74. Spektrum Wissensch. 1985, No. 12, p. 78.
- 16(a) E. Jostkleigrewe (Ed): Makromolekulare Chemie – Das Werk Hermann Staudingers in seiner heutigen Bedeutung, Schnell und Steiner. München 1967; (b) H. Morawetz, Angew. Chem. 99 (1987) 95; Angew. Chem. Int. Ed. Engl. 26 (1987) 93.
- 17 H. Morawetz: Polymers. The Origin and Growth of a Science, Wiley. Chichester, England 1985.
- 18(a) O. W. Websater, W. R. Hertler, D. Y. Sogah, W. B. Farnham, T. V. Rajan Baba, J. Am. Chem. Soc. 105 (1983) 5706; (b) M. T. Reetz, Angew. Chem. 100 (1988) 1026; Angew. Chem. Int. Ed. Engl. 27 (1988) 994.
- 19(a) T. Higashimura, S. Aoshima, M. Sawamoto, Makromol. Chem. Macromol. Symp. 13/14 (1988) 457; (b) Makromol. Chem. Macromol. Symp. 13/14 (1988) 513: (c) M. Zsuga, R. Faust, J. P. Kennedy, Polym. Bull. 21 (1989) 273.
- 20(a) R. B. Merrifield, G. Barany in E. Gross, J. Meienhofer (Eds.): The Peptides, Vol. 2, Academic Press, New York 1980; (b) R. B. Merrifield, Angew. Chem. 97 (1985) 801; Angew. Chrm. Int. Ed. Engl. 24 (1985) 799; (c) M. Bodanzky: Principles of Peptide Synthesis, Springer, New York 1984; (d) M. Bodanzky, A. Bodanzky: The Praclics of Peptide Synthesis, Springer, New York 1984.
- 21
H. G. Khorana in
Nobel Lectures: Physiology or Medicine
1963–1970,
American Elsevier, New York
1973. p. 341;
Angew. Chem.
81
(1969)
1027.
10.1002/ange.19690812404 Google Scholar
- 22 A term coined to denote synthetic mimicry of biological supramolecular structures with self-assembling or covalent fixed architectures.
- 23 N. Turro, Angew. Chem. 98 (1986) 872; Angew. Chem. Int. Ed. Engl. 25 (1986) 882.
- 24 J. H. Fendkr, Acc. Chem. Res. 13 (1980) 7.
- 25
F. H. Kohnke,
A. M. Z. Slawin,
J. F. Stoddart,
D. J. Williams,
Angew. Chem.
99
(1987) 941;
Angew. Chem. Int. Ed. Engl.
26
(1987) 892;
siche auch
F. H. Kohnke,
X. P. Mathias,
J. F. Stoddart,
Angew. Chem. Adv. Mater,
101
(1989) 1129;
Angew. Chem. Int. Ed. Engl. Adv. Mater.
28
(1989) 1103:
Adv. Mater.
1
(1989) 275.
10.1002/adma.19890010806 Google Scholar
- 26 G. Maier, S. Pfriem, U. Schäfer, R. Matusch, Angew. Chem. 90 (1978) 552; Angew. Chem. Int. Ed. Engl. 17 (1978) 520.
- 27 P. E. Eaton, T. W. Cole, J. Am. Chem. Soc. 86 (1964) 3157.
- 28(a) L. A. Paquette, Chem. Rev, 89 (1989) 1051; (b) J. P. Melder, R. Pinkos, H. Fritz, H. Prinzbach, Angew. Chem. 101 (1989) 314; Angew. Chem. Int. Ed. Engl. 28 (1989) 305.
- 29(a)
C. O. Dictrich-Buchecker,
J.-P. Sauvage,
Angew. Chem.
101
(1989) 192;
10.1002/ange.19891010214 Google ScholarAngew. Chem. Int. Ed. Engl. 28 (1989) 189; (b) D. M. Walba, Tetrahedron 41 (1985) 3161; (c) A. Nickon, E. F. Silversmith: Organic Chemistry: The Name Game, Pergamon Press, New York 1987; K. Mislow, Chemtracts-Org. Chem. 2 (1989) 151.
- 30 K. J. Niklas, Sci. Am. 254 (1986) No. 3. p. 78.
- 31(a) B. J. West, A. L. Goldberger, Am. Sci. 75 (1987) 354; (b) N. MacDonald: Trees and Networks in Biological Models, Wiley. New York 1983.
- 32 D'Arcy W. Thompson: On Growth and Farm, Cambridge University Press, Cambridge. England 1961.
- 33 J. M. Lehn: Science (Washington) 227 (1985) 849.
- 34
F. Vögtle,
H.-G. Löhr,
J. Franke,
D. Worsch,
Angew. Chem.
97
(1985) 721:
10.1002/ange.19850970904 Google ScholarAngew. Chem. Int. Ed. Engl. 24 (1985) 727.
- 35 F. Vögtle, E. Weber (Eds.): Host Guest Complex Chemistry I–III ( Top. Curr. Chem. 98 (1981)), Host Guest Complex Chemistry I–III ( Top. Curr. Chem. 101 (1982)), Host Guest Complex Chemistry I-III ( Top. Curr. Chem. 121 (1984)).
- 36 F. Vögtle, E. Weber (Eds.): Biomimetic and Bioorganic Chemistry I-III ( Top. Curr. Chem. 128 (1985)), ( Top. Curr. Chem. 132 (1986)), ( Top. Curr. Chem. 136 (1986)).
- 37
H. Ringsdorf,
B. Schlerb,
J. Venzmer,
Angew. Chem.
100
(1988) 118;
10.1002/ange.19881000111 Google ScholarAngew. Chem. Int. Ed. Engl. 27 (1988) 113.
- 38 J. H. Fendler: Membrane Mimetic Chemistry, Wiley, New York 1982.
- 39 T. Kunitake, Y. Okashata, J. Am. Chem. Soc. 99 (1977) 3860.
- 40 J.-H. Fuhrhop, J. Mathieu, Angew. Chem. 96 (1984) 124; Angew. Chem. Int. Ed. Engl. 23 (1984) 100.
- 41 D. Y. Takigawa, D. A. Tirrell, Macromolecules 18 (1985) 338.
- 42 J. H. Fendler, Chem. Rev. 87 (1987) 877.
- 43 P. J. Flory, J. Am. Chem. Soc. 63 (1941) 3083, 3091, 3096.
- 44 P. J. Flory, Ann. N. Y. Acad. Sci. 57 (1953) No. 4, p. 327.
- 45 P. J. Flory, J. Am. Chem. Soc. 74 (1952) 2718.
- 46 P. J. Flory: Principles of Polymer Chemistry, Cornell University Press, Ithaca, NY, USA 1953.
- 47 W. H. Stockmayer, J. Chem. Phys. 11 (1943) 45; J. Chem. Phys. 12 (1944) 125.
- 48 B. Zimm, W. H. Stockmayer, J. Chem. Phys. 17 (1949) 1301.
- 49 P. J. Flory, J. Rehner, J. Chem. Phys. 11 (1943) 512.
- 50 W. W. Graessley, Macromolecules 8 (1975) 185
- 51 W. W. Graessley, Macromolecules 8 (1975) 865.
- 52(a)
M. Gordon,
G. N. Malcolm,
Proc. R. Soc. (London) A
295
(1966) 29;
(b)
M. Gordon,
S. B. Ross-Murphy,
Pure Appl. Chem.
43 1:
(c)
M. Gordon,
G. R. Dobson,
J. Chem. Phys.
43
(1975) 35;
(d)
K. Dusak,
Makromol. Chem. Suppl.
2
(1979) 35;
10.1002/macp.1979.020021979103 Google Scholar(e) W. Burchard, Adv. Polym. Sci. 48 (1988) 1.10.1007/3-540-12030-0_1 Google Scholar
- 53(a) I. J. Good, Proc. Cambridge Phil. Soc. 45 (1948) 360: (b) Proc. R. Soc. (London) A 263 (1963) 54.
- 54 H. K. Hall, Jr., D. W. Polls, Polym. Bull. 17 (1987) 409.
- 55(a) R. A. Jacobson, J. Am. Chem. Soc. 54 (1932) 1513; (b) W. H. Hunter, G. H. Woolett, J. Am. Chem. Soc. 43 (1921) 135.
- 56 C. O. Beckman, Ann. N. Y. Acad. Sci. 57 (1953) 384.
- 57 S. Erlander, D. French, J. Polym. Sci. 20 (1956) 7.
- 58(a) W. Burchard, Macromolecules 5 (1972) 604; (b) W. Burchard, I. Kranztz, B. Pfannenmüller, Makromol. Chem. 150 (1971) 63.
- 59 E. Buhlein, W. Wehner, F. Vögtle, Synthesis 1978, 155.
- 60 D. A. Tomalia, 1st Society Polymer Science. Japan, Int. Polym. Conf., August 1984. Kyoto, Japan.
- 61 D. A. Tomalia, 6th Biennial Carl S. Marvel Symp, 19 March 1985, Tucson, AZ, USA.
- 62 D. A. Tomalia, H. Baker, J. R. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder, P. Smith, Polym. J. (Tokyo) 17 (1985) 117.
- 63 D. A. Tomalia, H. Baker, J. R. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder, P. Smith, Macromolecules 19 (1986) 2466.
- 64 D. A. Tomalia, V. Berry, M. Hall, D. M. Hedstrand, Macromolecules 20 (1987) 1164.
- 65
H. Hall,
A. Padias,
R. McConnell,
D. A. Tomalia,
J. Org. Chem.
52
(1987) 5305.
10.1021/jo00234a006 Google Scholar
- 66 D. A. Tomalia, J. R. Dewald, US Pat. 4507466 (1985); US Pat. 4558 120 (1985); US Pat. 4568737 (1986); US Pat. 4587329 (1986); US Pat. 4631 337 (1986); US Pat. 4694064 (1987); US Pat 4713975 (1987); US Pat. 4737550 (1988); US Pat. 4871 779 (1989); US Pat. 4857599 (1989).
- 67 D. A. Tomalia, M. Hall, D. M. Hedstrand, J. Am. Chem. Soc. 109 (1987) 1601.
- 68 P. G. de Gennes, H. J. Hervet, Phys. Lett. (Paris) 44 (1983) 351.
- 69 D. A. Tomalia, Bürgenstock Conf., May 3, 1987. Bürgenstock, Switzerland. See Nachr. Chem. Tech. Lab. 35 (1987) 693.
- 70(a) This term was coined in deference to their branched (dendritic = treelike) as well as their oligomeric nature, (b) The dendrimer generation numbering system used in earlier work [62–64, 67, 111] designated the first starbranched species derived from the initiator core as generation 1. The present system is preferred wherein that starbranched intermediate is designated generation O. thus making it consistent with the geometric progression (cf. Section 4.2.1). (c) R. E. Merrifield, H. E. Simmons (Eds.): Topological Methods in Chemistry, Wiley, New York 1989.
- 71 D. Cram, Science (Washington) 238 (1987) 612.
- 72 M. Maciejewski, J Macromol. Sci. Chem. A 17 (1982) 689.
- 73 R. G. Denkewalter, J. F. Kolc, W. J. Lukasavage, US Pat. 4410688 (1983): Chem. Abstr. 100 (1984) 103907p.
- 74(a) S. M. Aharoni, C. R. Crosby IIII, E. K. Walsh, Macromolecules 15 (1982) 1093; (b) S. M. Aharoni, N. S. Murthym, Polym. Commun. 24 (1983) 132.
- 75 G. R. Newkome, Z.-q. Yao, G. R. Baker, V. K-.Gupta, J. Org. Chem. 50, (1985) 2003.
- 76 G. R. Newkome, Z-q. Yao, G. R. Baker, V. K. Gupta, P. S. Russo, M. J. Saunders, J. Am. Chem. Soc. 108 (1986) 849.
- 77 G. R. Newkome, G. R. Baker, M. J. Saunders, P. S. Russo, V. K. Gupta, Z.-q. Yao, J. E. Miller, K. Bouillion, J. Chem. Soc. Chem. Comm. 1986, 752.
- 78 G. Cantor, Contributions to Transfinite Numbers, Dover Press. London 1915.
- 79 J. Gleick: Chaos: Making a New Science, Viking Penguin, New York 1987. p. 99.
- 80 B. B. Mandelbrot: Fractals: Form, Chance and Dimension, W. H. Freeman, New York 1977; The Fractal Geometry of Nature, W. H. Freeman, New York 1982.
- 81 B. H. Kaye: A Random Walk Through Fractal Dimensions, VCH Verlagsgesellschaft VCH Publishers, Weinheim/New York 1989.
- 82 See also the following communication in this issue: A. B. Blumen, H. Schnörer, Angew. Chem. 102 (1990) 158; Angew. Chem. Int. Ed. Engl. 29 (1990) 113.
- 83 A. Cheetham, K. McCormick (Smithsonian Institution. Washington, D. C., USA), personal communication.
- 84 M. Lewis, D. C. Rees, Science (Washington) 230 (1985) 163.
- 85 P. B. Smith, S. J. Martin, M. J. Hall, D. A. Tomalia in J. Mitchell, (Ed.): Applied Polymer Analysis and Characterization, Hanser, München/New York 1987, p. 357.
- 86 F. M. Menger, Top. Curr. Chem. 136 (1986) 1.
- 87 D. A. Tomalia, P. Kirchoff, D. Downing, unpublished results.
- 88 O. C. Dermer, G. E. Ham: Ethyleneimine and Other Aziridines, Academic Press, New York 1969.
- 89 D. A. Tomalia, G. Killat (“ Alkyleneimine Polymers”) in Encyclopedia of Polymer Science and Engineering, 2nd ed, Vol. 1, Wiley, New York 1985.
- 90 D. M. Hedstrand, P. Meister, D. A. Tomalia, unpublished results.
- 91 A. Bashir-Hashemi, H. Hart, D. L. Wart, J. Am. Chem. Soc. 108 (1986) 6675.
- 92 H. Hart, A. Bashir-Hashemi, J. Luo, M. Meador, Tetrahedron 42 (1986) 1641.
- 93 H. Hart, personal communication.
- 94 A. Padias, H. Hall, D. A. Tomalia, Polym. Prepr. Am. Chem. Soc. Div. Polym. Chem. 30 (1989) 119.
- 95 M. N. Bochkarev, Organomet. Chem. (USSR) 1 (1988) 115.
- 96 Y. H. Kim, O. W. Webster, Polym. Prep. Am. Chem. Soc. Div. Polym. Chem. 29 (1988) 310.
- 97(a) S. J. Weiner, P. A. Kollman, D. A. Case, U. C. Singh, C. Ghio, G. Alagona, S. Profeta, Jr., P. Weiner, J. Am. Chem. Soc. 106 (1984) 765; (b) POLYGRAF is an interactive molecular mechanics/graphics program available from Molecular Simulations, Inc. Pasadena, CA, USA. All simulations were performed on DEC Vax 8650 and Alliant FX8/8 computers. Structures were displayed on Evans & Sutherland PS330 and PS390 series graphics systems.
- 98 F. M. Richards, Annu. Rev. Biophys. 6 (1977) 151.
- 99 J. L. Pascual-Ahuir, E. Silla, QCPE Program No. 554, Quantum Chemistry Program Exchange Center, Bloomington, IN, USA.
- 100 D. A. Tomalia, S. Edwards, D. M. Hedstrand, P. Meister, unpublished results.
- 101 L. R. Wilson, D. A. Tomalia, Polym. Prepr. Am. Chem. Soc. Div. Polym. Chem 30 (1989) 115.
- 102(a) D. Meltzer, D. A. Tirell, unpublished results, b P. Smith, unpublished results.
- 103 R. D. Hester, P. H. Mitchell, J. Polym. Sci. Chem. Ed. 18 (1980) 1729.
- 104 C. Milstein, Angew. Chem. 97 (1985) 819; Angew. Chem. Int. Ed. Engl. 24 (1985) 816.
- 105 A. Numen, S. Demotz, G. Corradin, H. Binz, H. R. Bosshard, Science (Washington) 235 (1987) 780.
- 106 A. G. Amit, R. A. Mariuzza, S. E. V. Phillips, R. J. Poljak, Science (Washington) 233 (1985) 747.
- 107 M. Lewis, D. C. Rees, Science (Washington) 230 (1985) 1163.
- 108 J. F. Leszczynski, G. D. Rose, Science (Washington) 234 (1986) 849.
- 109(a) M. Daoud, J. P. Cotton, J. Phys. (Paris) 43 (1982) 531; (b) T. M. Birshtein, E. B. Zhulina, Polymer 25 (1984) 1453.
- 110 A. Miyake, K. F. Freed, Macromolecules 16 (1983) 1228.
- 111 A. M. Naylor, W. A. Goddard III, G. Keifer, D. A. Tomalia, J. Am. Chem. Soc. 111 (1989) 2339.
- 112(a) S. S. Kurtz, Jr., A. L. Ward, J. Franklin Inst. 222 (1936) 563; J. Franklin Inst. 224 (1937) 583, 697; (b) A. L. Ward, S. S. Kurtz, Jr., W. H. Fuleveiler in A. E. Dunstan, A. W. Nash, B. T. Brooks, N. T. Tizard (Eds.): Science of Petroleum. Vol. 2, Oxford University Press, London 1938, p. 1172; (c) S. S. Kurtz, Jr., A. Sankin in A. Farkas (Ed.): Physical Chemistry of Hydrocarbons. Vol. 2, Academic Press, New York 1953.
- 113 L. Stryer: Biochemistry, 2nd ed. W. H. Freeman, New York 1981, p. 724.
- 114 J. W. Erickson, A. M. Silva, M. R. N. Muthy, I. Fila, M. G. Rossman, Science (Washington) 229 (1985) 625.
- 115 F. M. Brodsky, Science (Washington) 242 (1988) 1396.
- 116 It is well known that classical systems of particles with purely repulsive two-body interactions freeze into an ordered crystalline-like phase if they are sufficiently dense, (a) M. Ross, Contemp. Phys. 12 (1971) 333; (b) M. N. V. Temperleg, J. S. Rowlinson, G. S. Rushbrooke (Eds): Physics of Simple Liquids, North-Holland, Amsterdam 1968.
- 117 R. Kopelman, Science (Washington) 241 (1988) 1620.
- 118 S. E. Friberg, M. Podzimek, D. M. Hedstrand, D. A. Tomalia, Mol. Cryst. Liq. Cryst. 164 (1988) 157–165.
- 119
F. Vögtle,
E. Weber,
Angew. Chem.
86
(1974) 896;
10.1002/ange.19740862407 Google ScholarAngew. Chem. Int. Ed. Engl. 13 (1974) 814.
- 120 C. J. Suckling, J. Chem. Soc. Chem. Commun. 1982, 661.
- 121 See [86], particularly chapters 2–4.
- 122 H. Sigel, R. B. Martin, Chem. Rev. 82 (1982) 385.
- 123 B. Helmer, D. A. Tomalia, unpublished results.
- 124 D. S. Edwards, C. W. Jung, D. A. Tomalia, unpublished results.
- 125 I. A. Tomlinson, M. J. Fazio, unpublished results.
- 126 C. Tanford: The Hydrophobic Effect: Formation of Micelles and Biological Membranes, 2nd ed., Wiley-Interscience, New York 1980.
- 127 D. J. Mitchell, B. W. Ninham, J. Chem. Soc. Faraday Trans. 2 1981, 601.
- 128 D. A. Tomalia, D. A. Kaplan, J. W. Kruper, R. C. Cheng, I. A. Tomlinson, M. J. Fazio, D. M. Hedstrand: PCI Int. Appl. W0881, 178; Chem. Abstr. 110 (1989) 150378x.
- 129 J. P. Tam, Proc. Natl. Acad. Sci. USA 88 (1988) 5409.
- 130 K. J. Chang, W. Pugh, S. G. Blanchord, J. McDermed, J. P. Tam, Proc. Natl. Acad. Sci. USA 85 (1988) 4929.
- 131 D. N. Posnett, H. McGrath, J. P. Tam, J. Biol. Chem. 264 (1988) 1719.
- 132 A. G. Cairns-Smith: Sci. Am. 253 (1985) No. 6, p. 90; Spektrum Wiss. 1985, No. 8, p. 82.
- 133 A. G. Cairns-Smith: Genetic Takeover and the Mineral Origins of Life, Cambridge University Press, Cambridge, England 1982.
- 134 R. Dawkins, New Scientist 25 (1986) 24.
- 135 K. Verner, G. Shatz, Science (Washington) 241 (1988) 1307.
- 136 W. J. Gehring, Sci. Am. 253 (1985) No. 10, p. 153; D. R. Kaplan, Sci. Am. 249 (1983) No. 7, p. 98.
- 137(a) J. Darnell, H. Lodish, D. Baltimore: Molecular Cell Biology, Scientific American Books, New York 1986, chapt. 22, pp. 987 ff; (b) I. G. David, T. D. Sargent, Science (Washington) 240 (1986) 1443.
- 138 D. A. Tomalia et al., unpublished results.
- 139 D. J. Cram, Science (Washington) 219 (1983) 1177.
- 140 J. L. Marx, Science (Washington) 222 (1983) 1109.
- 141 J. H. White, N. R. Cozzarelli, W. R. Bauer, Science (Washington) 241 (1988) 323.
- 142 See[137a], p. 1086.
- 143 D. W. Tank, M. Sugimori, J. A. Connor, R. R. Llinás, Science (Washington) 242 (1988) 773.
- 144 R. A. Lerner, K. D. Janda, D. Schloeder, S. J. Benkovic, Science (Washington) 241 (1988) 323.
- 145Review: P. G. Schultz, Angew. Chem. 101 (1989) 1336; Angew. Chem. Int. Ed. Engl. 28 (1989) 1283.
- 146 T. C. Lubensky, P. A. Pincus, Phys. Today 37 (1984) No. 10. p. 44.
- 147 T. P. Martin, Angew. Chem. 98 (1986) 197; Angew. Chem. Int. Ed. Engl. 25 (1988) 197.
- 148 J. M. Lehn, A. Rigault, Angew. Chem. 100 (1988) 1121; Angew. Chem. Int. Ed. Engl. 27 (1988) 1095, and references therein.
- 149 Spheroidal cancer tumor growth is analyzed in this fashion and exhibits strong analogies to spherical dendrimers, W. Duchting, T. Vogelsacnger, Bio Systems 18 (1985) 79; R. M. Sutherland,. Science (Washington) 240 (1988) 177.
- 150 E. J. Goethals (Ed.): Telechelic Polymers: Synthesis and Applications, CRC Press, Boca Raton, FL, USA 1989.
- 151(a) A. Halperinm Macromolecules 20 (1987) 2943; (b) C. Marques, J. F. Joanny, L. Leibler, Macromolecules 21 (1988) 1051; (c) A. Halperin, Macromolecules 22 (1989) 3806.
- 152 See [137a]. pp. 774 ff.
- 153 J.-H. Fuhrhop, H.-H. David, J. Mathieu, U. Liman, H.-J. Winter, E. Bockema, J. Am. Chem. Soc. 108 (1986) 1785.
- 154(a) C. D. Gutsche, L.-G. Lin, Tetrahedron 42 (1986) 1633; (b) E. T. Jarvi, H. W. Whitlock, J. Am. Chem. Soc. 1114 (1982) 7196.
- 155 L. Pauling, Chem. Eng. News 24 (1946) 1375; Am. Sci. 36 (1948) 51.
- 156 H. Hoffmann, G. Ebert, Angew. Chem. 100 (1988) 933; Angew. Chem. Int. Ed. Engl. 27 (1988) 902.
- 157 E. G. Mazur: Graphic Representation of the Periodic System During One Hundred Years, The University of Alabama Press, AL, USA 1974; I. Hargittai, M. Hargittai: Symmetry Through the Eyes of a Chemist, VCH, Weinheim/New York 1986, pp. 5f.
- 158 D. A. Tomalia, D. M. Hedstrand, L. R. Wilson in Encyclopedia of Polymer Science and Engineering. 2, Wiley, New York. 1990. pp. 46–92.
- 159 A. A. Berlin, N. G. Matveyeva, J. Polym. Sci. Macromol. Rev. 12 (1977) 1; J. Polym. Sci. Macromol. Ret: 15 (1980) 107.
- 160 P. J. Flory, Br. Polym. J. 17 (1985) 96.
- 161 P. Hodge, D. C. Sherrington (Eds): Polymer Supported Reactions in Organic Synthesis, Wiley, New York 1980.
- 162 See [137a], pp. 816f.
- 163
S. Borman,
Chem. Eng. News
67
(1989) No. 15 (April 10,
1989), p. 25.
10.1145/71573.71574 Google Scholar
- 164(a) Cationic surfactants have been successfully organized and characterized on the surfaces of anionically functionalized dendrimers. A “cooperative effect” was noted for higher-generation PAMAM dendrimers (generations 4.5–9.5) according to photoluminescence probe techniques, b) Generation-dependent morphologies consistent with [111] were corroborated by probing with photoinduced electron-transfer reactions on the dendrimer surfaces: N. D. Turro, G. Caminati, D. A. Tomalia, Macromolecules (1990), in press.
- 165 J. P. Crutchfield, J. D. Farmer, N. H. Packard, R. S. Shaw, Sci. Am. 255 (1986) No. 1, p. 46.
- 166 One might argue that artificial protein synthesis as described by M. Mutter [4b] is one of the first efforts in this direction.
- 167 This has been demonstrated for micron-sized particles referred to as Janus beads: (a) E. Raphael, C. R. Acad. Sci. Paris 307 (1988) 12; (b) C. Casagrande, M. Veyssie, C. R. Acad. Sci. Paris 306 (1988) 1423.
- 168 M. Maclean, S. P. Gregory, R. A. Flavell (Eds.): Eukaryotic Genes, Their Structure, Activity and Regulation, Butterworth & Co., London/Boston 1983.
- 169 W. Ostwald, M. H. Fischer (Eds.): Theoretical and Applied Colloid Chemistry: “The World of Neglected Dimensions”, Wiley 1917.
- 170 M. A. Duncan, D. H. Rouvray, Sci. Am., No. 12 (1989), p. 110.