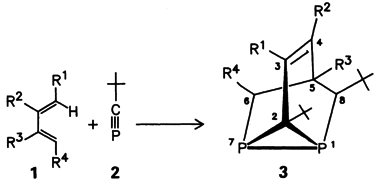
Mono- and Diphosphatricyclo [3.2.1.02,7]oct-3-enes: Synthesis from 1,3- and 1,4-Dienes and a Stable Phosphaalkyne†
Phosphorus Compounds with Unusual Coordination, Part 23. This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.—Part 22: W. Rösch, M. Regitz, Synthesis 1987, 689.
Graphical Abstract
Via a sequence consisting of a Diels-Alder reaction, an ene reaction, and an intramolecular [4 + 2] cycloaddition, the 1,7-diphosphatricyclo [3.2.1.02,7]octenes 3 are formed from 1,3-dienes 1 and the phosphaalkyne 2 (molar ratio 1:2) in a one-pot procedure. Both the ene reaction of phosphaalkynes and the intramolecular cycloaddition reaction of phosphaalkenes are new. R1 = H, R2–R4 = 1 × Me, 2 × H.