Generation and Interception of 1-Oxa-3,4-cyclohexadiene†
This work was supported by the Fonds der Chemischen Industrie.
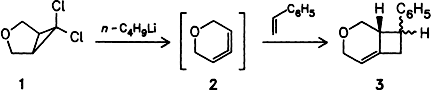
Graphical Abstract
The title compound 2 could be the most strained monocyclic allene. It is generated from 1 by reaction with n-butyllithium and can be trapped with a series of alkenes. Thus, with styrene the diastereomeric [2 + 2] adducts 3 are formed. 2 reacts with 1,3-dienes to give [2 + 2]- and [2 + 4]-adducts, and, in the absence of reactive partners, 3-n-butyl-2,4-pentadien-1-ol is formed from 2 upon addition of n-BuLi.