Optically Active α-Arylglycine Esters by Friedel-Crafts Alkylation with the Chiral Cation of the Bislactim Ether of cyclo-(L-Val-Gly)†
Corresponding Author
Prof. Dr. Ulrich Schöllkopf
Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Ulrich Schöllkopf, Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Ernst Egert, Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Search for more papers by this authorDipl.-Chem. Sabine Grüttner
Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Search for more papers by this authorDr. Ralf Anderskewitz
Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Search for more papers by this authorCorresponding Author
Dr. Ernst Egert
Institut für Anorganische Chemie der Universität, Tammannstrasse 4, D-3400 Göttingen (FRG)
Ulrich Schöllkopf, Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Ernst Egert, Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Search for more papers by this authorMichael Dyrbusch
Institut für Anorganische Chemie der Universität, Tammannstrasse 4, D-3400 Göttingen (FRG)
Search for more papers by this authorCorresponding Author
Prof. Dr. Ulrich Schöllkopf
Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Ulrich Schöllkopf, Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Ernst Egert, Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Search for more papers by this authorDipl.-Chem. Sabine Grüttner
Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Search for more papers by this authorDr. Ralf Anderskewitz
Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Search for more papers by this authorCorresponding Author
Dr. Ernst Egert
Institut für Anorganische Chemie der Universität, Tammannstrasse 4, D-3400 Göttingen (FRG)
Ulrich Schöllkopf, Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Ernst Egert, Institut für Organische Chemie der Universität, Tammannstrasse 2, D-3400 Göttingen (FRG)
Search for more papers by this authorMichael Dyrbusch
Institut für Anorganische Chemie der Universität, Tammannstrasse 4, D-3400 Göttingen (FRG)
Search for more papers by this authorAsymmetric syntheses via Heterocyclic Intermediates, Part 35.–Part 34: U. Schöllkopf, W Kühnle, E. Egert, M. Dyrbusch, Angew. Chem. 99 (1987) 480; Angew. Chem. Int. Ed. Engl. 26 (1987) 480.
Graphical Abstract
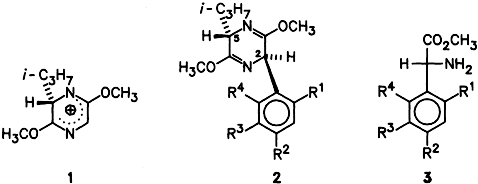
The chiral non-racemic glycine cation equivalent 1 enabled the synthesis of the previously unknown methyl (R)-α-arylglycinate 3. The Friedel-Crafts adducts 2 could be hydrolyzed without epimerization or racemization with 2 equivalents of 0.1 N HCl. α-Arylglycines are of particular interest as pharmacophoric building blocks of drugs. (R1–R4 H, OEt, OMe.)
References
- 1 Reviews: U. Schöllkopf, Pure Appl. Chem. 55 (1983) 1799; Chem. Scr. 25 (1985) 105; in H. Prinzbach, G. Schill, K. Streith (Ed.): Organic Synthesis: An Interdisciplinary Challenge, Blackwell, Oxford, 1985, p. 101ff.
- 2 For achiral glycine-cation equivalents, see D. Ben-Ishai, S. Hirsch, Tetrahedron Lett. 24 (1983) 955 and other publications of this group, T. Shono, Tetrahedron 40 (1984) 811; R. Kober, W. Hammes, W. Steglich, W. D. Bennet, M. J. O'Donell, R. L. Polt, Tetrahedron Lett. 26 (1985) 695; Y. Ozaki, T. Iwasaki, H. Harikawa, M. Miyoshi, K. Madzumoto, J. Org. Chem. 44 (1979) 391; for chiral glycine-cation equivalents, see R. Kober, K. Papadopoulos, W. Miltz, D. Enders. W. Steglich, H. Reuter, H. Puff, Tetrahedron 41 (1985) 1693; P. J. Sinclair, D. Zhai, J. Reibenspies, R. M. Williams. J. Am. Chem. Soc. 108 (1986) 1103.
- 3 For example, D-phenylglycine or D-p-hydroxyphenylglycine as side chains in the semisynthetic penicillins, ampicillins. or amoxycillins.
- 4(a)
K. Weinges,
H. Brachmann,
P. Stahnecker,
H. Rodewald,
M. Nixdorf,
H. Irngartinger,
Liebigs Ann. Chem.,
1985, 566;
10.1002/jlac.198519850318 Google Scholar(b) K. Weinges, G. Brune, H. Droste. Liebigs Ann. Chem., 1980 212;10.1002/jlac.198019800205 Google Scholar(c) S. Yamada, S. Hashimoto, Chem. Lett., 1976, 921.
- 5
U. Schöllkopf,
H.-J. Neubauer,
M. Hauptreif,
Angew. Chem.
97
(1985) 1065;
10.1002/ange.19850971223 Google ScholarAngew. Chem. Int. Ed. Engl. 24 (1985) 1066. The procedure is carried out initially as described starting from 1 (0.92 g, 5.0 mmol). After reaction with hexachloroethane, the tetrahydrofuran is removed under vacuum, 2 is extracted with ca. 40 mL of petroleum ether, the LiCl is filtered off, the petroleum ether is removed under vacuum, and 2 (5.0 mmol) is dissolved in 10 mL of dichloromethane.
- 6
U. Schöllkopf,
U. Groth,
C. Deng,
Angew. Chem.
93
(1981) 791;
10.1002/ange.19810930913 Google ScholarAngew. Chem. Int. Ed. Engl. 20 (1981) 798; 1 is commercially available: Merck-Schuchardt, D-6100 Darmstadt (FRG), MS-Info 85–14.
- 7 Nonactivated arenes do not react.
- 8 Preparative GC: Varian 220, 2.2-m glass column, OV-220 on Chromosorb-W (AW-DMCS, 60/80 mesh), p = 14–17 N· Cm−2 (T = 160 °C), H2 as carrier gas. Relative retention times: trans-6c: cis-6c:6d = 1: 1.64: 1.74.
- 9 Varian 3700, Durabond-1; 30 m, H2 as carrier gas.
- 10 Space group P21, a = 1376.7(l), b = 1026.4(1), c = 1435.6(1) pm, β = 114.66(1)°, V = 1.844 nm3, Z = 4, μ = 0.07 nm−1 (MoKα); crystal dimension; 0.7 × 0.6 × 0.5 mm3; 3961 measured intensities, 2ωmax = 50°, 3448 unique reflections with |F| > 3σ( F) used for the structure solution (direct methods) and refinement; C, N, and O atoms refined anisotropically, H atoms localized by difference electron density determinations and refined with a “riding” model. R = 0.037 (Rw = 0.040, w−1 = σ(F2) + 0.0006ċ F2). Further details of the crystal structure investigation may be obtained from the Fachinformationszentrum Energie, Physik, Mathematik GmbH, D-7514 Eggenstein-Leopoldshafen 2 (FRG), on quoting the depository number CSD-52388, the names of the authors, and the journal citation.
- 11 It is surprising that the compounds 6a–c, which contain an aryl ring at C2 capable of undergoing conjugation, do not rapidly tautomerize in acidic medium to enamines and thus undergo epimerization at C2. As shown by the X-ray structure analysis (Fig. 1), the aryl ring is orthogonal to the heterocycle. This conformation is presumably strongly fixed by the o-substituled aryl rings ( 6a, b, 7). Thus, no π conjugation is possible between the aryl ring and the potential enamine double bond.
- 12 R. F. Eizemberger, A. S. Ammons, Org. Prep. Proced. Int. 8 (1976) 149.
- 13 The use of chloroform instead of dichloromethane results in better separation of the phases upon extraction.
- 14 If necessary, 8a can be separated by low-pressure chromatography (silica gel, ether/petroleum ether = 1/3). However, 6c/d thereby epimerize to a small extent.
- 15 Determined with 40-mol% Eu(tfc), at the 1H-NMR signal of the ester OCH3 group (CDCl3); >95% was assumed when the signals were shifted but no splitting occurred.