Electrostatics, the Chemical Bond and Molecular Stability
Graphical Abstract
Abstract
Electrostatic models of the chemical bond are based on the Virial Theorem and hold promise for providing a reliable and accurate method for predicting heats of formation of molecules and free radicals. The Principle of Alternating Polarity which states that those compounds are most stable in which atoms of opposite polarity are bonded is shown to be quantitatively described by electrostatic models. Current fixed partial-charge models account for ΔH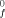 of hydrocarbon molecules and radicals. With inclusion of polarization effects, whose energies are small, they also account for the dipole moments in hydrocarbons. A brief account is given of a more general model with significant polarization interaction which is under development and which appears to be able to account for both ΔH
of hydrocarbon molecules and radicals. With inclusion of polarization effects, whose energies are small, they also account for the dipole moments in hydrocarbons. A brief account is given of a more general model with significant polarization interaction which is under development and which appears to be able to account for both ΔH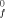 and dipole moments of polar molecules.
and dipole moments of polar molecules.



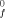 and dipole moments of molecules and radicals.
and dipole moments of molecules and radicals.
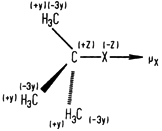
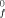 since the heats of combustion of graphite and H2 are not known with greater precision. The absolute accuracy with which these quantities are known cannot exceed this. There are, unfortunately, very few independent experimental sources of data with which to compare these combustion derived data and it is our feeling that the accuracy of the data is not better than ±0.1 kcal per C atom in hydrocarbons.
since the heats of combustion of graphite and H2 are not known with greater precision. The absolute accuracy with which these quantities are known cannot exceed this. There are, unfortunately, very few independent experimental sources of data with which to compare these combustion derived data and it is our feeling that the accuracy of the data is not better than ±0.1 kcal per C atom in hydrocarbons.
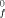 ) are given by ΔH(XA/XA) + ΔH(XB/XB) −2ΔH(XA/XB) where ΔH(XA/XB) represents the energy of interaction of the bond X
) are given by ΔH(XA/XA) + ΔH(XB/XB) −2ΔH(XA/XB) where ΔH(XA/XB) represents the energy of interaction of the bond X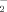 .
.

