Lithium 7bH-Indeno [1,2,3-jk] fluorenide, an Organolithium Compound Having a “Sandwich” Structure†
Strained Alkyl-Aromatic Systems, Part 4. This work was supported by the Fonds der Chemischen Industrie.—Part 3: P. Luger, Ch. Tuchscherer, M. Grosse, D. Rewicki, Chem. Ber. 109, 2596 (1976).
Graphical Abstract
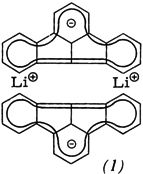
The organolithium compound (1) displays a variety of special features. Thus (1) is the first Li salt of a hydrocarbon having extreme charge delocalization in the carbanion, which crystallizes without heteroatom bases. Compound (1) is the first example of a substance in which Li is linearly coordinated with two hexahapto-bonded six-membered ring ligands. Moreover, there is no preferential orientation of the Li atoms on the C centers having the highest charge density.