Reductive N2 Cleavage and Nitride Insertion Reactivity at Molybdenum Complexes Supported by a Rigid PNP Pincer
Abstract
Metal-nitride species represent key reactive intermediates in reductive N2 cleavage and functionalization. Toward a better understanding of nitride transfer reactivity, we present a series of Mo-nitride complexes supported by a rigid diphosphino-acridane pincer ligand. Notably, the stepwise reduction of a MoIVCl3 complex under N2 allows the isolation of a bridging diazenido intermediate as well as a dinuclear Mo-nitride species (4) accommodating a bridging N2 moiety. This is the first example of a Mo(IV) nitride-N2 species, and extensive 15N-labeling studies were performed to characterize the bridging dinitrogen, diazenido, and nitride moieties. The structure of 4 adopts two orthogonal π-backbonding interactions between N2 and two structurally rigidified MoIV(N) fragments featuring a filled dπ orbital. Effective π-backbonding from such a MoIV(N) fragment leads to the isolation of PMe3, CO, and CNtBu substituted analogues. Further, reactivity of the Mo-nitride species with π-acidic CO and isocyanide leads to the formation of MoII-isocyanate and -carbodiimide species, respectively. Computational studies suggest that nitride carbonylation proceeds via an associative nitride insertion pathway, encouraging further studies in direct N2 fixation to value-added products via nitride functionalization.
Introduction
Metal nitrides have received significant attention as key reactive intermediates involved in N2 activation and cleavage, which is a critical process in the global nitrogen cycle and the production of nitrogen-containing chemicals.[1-4] In particular, molybdenum complexes have been extensively investigated for N2 reduction, taking advantage of the wide range of oxidation states available at Mo.[5-7] In general, reduced molybdenum centers bind and activate N2, with subsequent electron transfer steps culminating in the formation of high-valent Mo-nitride multiply bonded species.[8-11] Considering the electron-rich nature of the nitride moiety (N3−), the majority of structurally characterized Mo-nitrides can be assigned as MoVI≡N (95 examples), followed by MoIV≡N (30 examples) and MoV≡N (19 examples).[12] Notably, Mo-nitrides can be derived from dimeric Mo-N2 complexes supported by several pincer ligands.[13, 14] In such systems, the N─N bond cleavage may occur spontaneously or may be induced via protonation or photolysis (Figure 1). Alternatively, Mo-nitrides can be obtained via reductive N2 cleavage, while experimental data regarding the bridging-N2 intermediate remains rare.[15, 16] Given this propensity of Mo and related systems to activate N2 to form metal-nitride species, several methodologies have been developed for their conversion to value-added products involving silanes, CO, and alkynes as substrates.[17-20] In this regard, further developments in the functionalization of metal-nitride species derived from N2 are highly desirable.
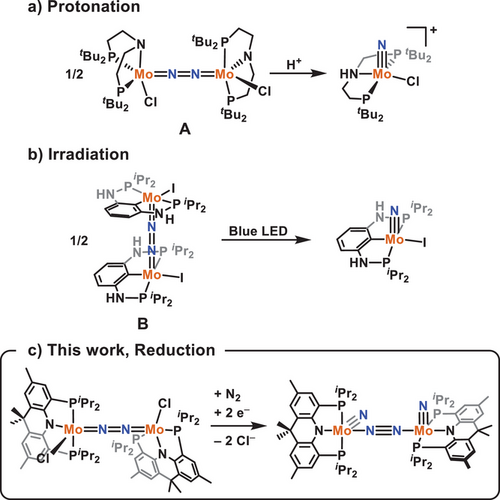
Herein, we report the stepwise reductive cleavage of N2 at a Mo center supported by a rigid acridane–diphosphine pincer ligand, resulting in the formation of a bridging diazenido and an unprecedented dinuclear nitride–dinitrogen species (N)Mo-N2-Mo(N). While such a dimolybdenum nitride dinitrogen species has been proposed as a key intermediate in the generation of monomeric MoIV≡N species within the catalytic cycle of ammonia synthesis, direct experimental evidence for its formation was limited to mass spectrometry.[21] The bridging diazenido and dinitrogen complexes were fully characterized by single crystal X-ray crystallography and 15N NMR spectroscopy. The isolation of the dinitrogen species was facilitated by the presence of two orthogonal π-backbonding interactions between N2 and two structurally rigidified MoIV(N) fragments featuring a filled dπ orbital. Its effective π-backbonding leads to the isolation of PMe3, CO, and CNtBu substituted analogues, highlighting an unusual, low-valent-like character of the MoIV(N) fragment. Further, reactivity of the Mo-nitride species with CO and isocyanide leads to the formation of MoII-isocyanate and -carbodiimide species, respectively. Computational studies suggest that the nitride carbonylation reaction proceeds via an associative nitride insertion pathway.
Results and Discussion
Reductive N2 Cleavage and 15N Labeling Studies
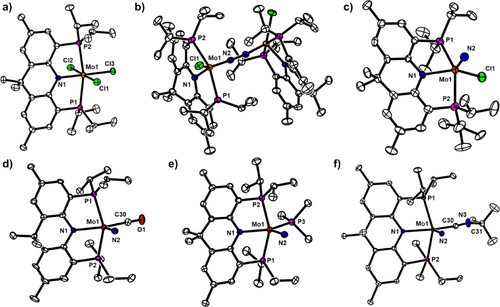
Initial metalation studies were performed to obtain a molybdenum chloride species amenable to reductive N2 cleavage. Treatment of the acridane–diphosphine ligand (acriPNP)H[22] with Mo(Cl)4(THF)2[23] and NEt3 in THF led to the formation of the trichloride species (acriPNP)Mo(Cl)3 (1), isolated as a dark purple powder in 65% yield (Scheme 1). Complex 1 exhibits paramagnetically shifted 1H NMR resonances in toluene-d8, and the solution magnetic moment of µeff = 2.46 µB is consistent with an S = 1 ground state arising from a MoIV d2 center. The crystal structure of 1 (Figure 3a) reveals an octahedral geometry around the Mo center, featuring a rigid acriPNP moiety binding in a strictly meridional fashion.[24] Subsequent addition of 2 equiv. of KC8 to a solution of 1 in THF at −78 °C resulted in a gradual color change from dark purple to yellow–brown to deep red and the formation of a dimeric species [(acriPNP)Mo(Cl)]2(µ-N2) (2), isolated as a dark red solid in 74% yield (Scheme 1). The 31P NMR spectrum of 2 displays two doublets at 56.5 and 58.3 ppm (2JPP = 145 Hz). The crystal structure of 2 (Figure 3b) features a dinuclear Mo═N═N═Mo motif with a Mo─N distance of 1.797(3) Å and a N─N distance of 1.255(5) Å (Table 1), consistent with previously reported dimolybdenum bridging diazenido species.[9, 13, 14, 25, 26] The degree of N2 activation is related to the inversely correlated Mo─N and N─N distances, and a survey of all crystallographically characterized Mo2(µ-N2) complexes reveals respective distances in the range of 1.77–2.18 Å and 1.12–1.27 Å (Figure S1).[12] Thus, 2 is among one of the most activated Mo-diazenido species. Notably, the Mo─N1 distance to the acridane motif is much longer, at 2.059(2) Å, indicating a substantial multiple bonding character between the Mo center and the diazenido moiety. Complex 2 was further characterized by 15N NMR spectroscopy. The 15N2 isotopologue 2–15N2 was prepared by reduction of 1 with KC8 under an 15N2 atmosphere. The 15N NMR spectrum of 2–15N displays a triplet at 431.2 ppm (JNP = 2.7 Hz), in close resemblance to analogous molybdenum-diazenido species A and B (Figure 1) exhibiting 15N resonances at 448.7 and 422.4 ppm, respectively.[13, 14] Finally, the Raman spectrum of 2 displays a N═N stretching band at 1361 cm−1 (KBr, Δ(15N2) = 38 cm−1), fully validating the activation of N2 to a bridging diazenido species with a formal oxidation state of [(acriPNP)MoIII(Cl)]2[µ-N22−].

| Parameter | dMo–N(PNP) | dMo–Nitride | dMo–N2 | dN–N or C–O | τ5 |
|---|---|---|---|---|---|
| 1 | 2.036(2) | – | – | – | – |
| 2 | 2.059(2) | – | 1.797(3) | 1.255(5)a) | 0.476 |
| 3 | 2.152(4) | 1.652(6) | – | – | 0.157 |
| 4 |
2.179(6) 2.219(6) |
1.646(7) 1.681(2) |
1.969(7) 2.047(8) |
1.18(1)b) | 0.036 |
| 5-COc) |
2.193(2) 2.198(2) |
1.666(3) 1.666(3) |
– |
1.151(4) 1.152(4) |
0.141 0.141 |
| 5-PMe3 | 2.233(3) | 1.666(3) | – | – | 0.176 |
| 5-CNtBuc) | 2.205(1) | 1.663(1) | – | – | 0.078 |
- a) dN2–N2.
- b) dN3–N6.
- c) Two molecules in the asymmetric unit.
The electronic structure of 2 was further studied by density functional theory (DFT) calculations. Results reveal that 2 adopts a singlet ground state, in agreement with experimental data.[27] Further inspection reveals that the HOMO-4 and HOMO-5 of 2 represent the π-backbonding interactions between the Mo centers and the π* orbital of the N2 moiety (Figure S98). Time-dependent DFT (TD-DFT) calculations indicate that the intense absorption band at 298 nm (48,000 M−1 cm−1) in the UV–vis absorption spectrum of 2 (Figure S100) can be assigned to the transition from the HOMO-7 to the LUMO+2. This represents a transition from a Mo-based orbital to the σ* orbital of N2, suggesting that UV-irradiation of 2 could induce N2 cleavage. Accordingly, irradiation of 2 with 365 nm light for 94 h in C6D6 in a quartz Schlenk tube resulted in a color change from red to green to give a product in 80% yield (Scheme 1). The resulting paramagnetic product was identified as (acriPNP)Mo(N)(Cl) (3). The Mo─N bond length of 1.655(7) Å indicates the formation of a Mo-nitride triple bond (Figure 3c). This transformation represents a net transfer of two electrons from each Mo center to fully cleave the N─N bond, resulting in the formation of a MoV-nitride species. Photolysis of 2–15N2 leads to the formation of the 15N2 isotopologue 3–15N, featuring a bathochromic shift of the Mo≡N stretching band from 1029 cm−1 in 3 to 1005 cm−1 in 3–15N. While chemical reduction of 3 leads to chloride extrusion and N2 coordination (vide infra), the cyclic voltammogram of 3 under N2 or Ar shows a quasi-reversible MoIV/V couple at −1.84 V versus Fc0/+ (Figure S95).
The structure of 2 was further compared to the bridging diazenido species A reported by the Schneider group.[13] While seemingly similar, the Mo─Namide distance of 1.917(7) Å in A is significantly shorter than the Mo─Nacridane distance of 2.059(2) Å in 2. This is related to the greater basicity of the dialkylamido moiety in A, which may influence subsequent reductive cleavage of N2. According to Schneider's report, the central N-donor engages in π-bonding with Mo and destabilizes the unoccupied frontier orbitals, hindering reductive processes. Protonation on the central N-donor breaks this π-bonding interaction with Mo, facilitating occupation of the σ* orbital between the two nitrogen atoms and ultimately leading to N─N bond cleavage. In the case of 2, the 2p orbital of the central N is delocalized within the planar acridane moiety of the acriPNP ligand, resulting in a relatively lower LUMO energy manifested in its reduction potential around −2.2 V versus Fc0/+ (Figure S92). Similar effects were observed in a related the (PNP)Re species.[28, 29] With this in mind, we explored reductive N2 cleavage pathways from 2. Treatment of 2 with 2 equiv. of NaC10H8 in THF under N2 leads to the formation of [(acriPNP)Mo(N)]2(µ-N2) (4), isolated as an orange–brown solid in 31% yield (Scheme 1). This is an interesting result, as the reduction of 2 resulted in the formation of the first example of a metal nitride–dinitrogen species obtained via reductive N2 cleavage. In addition to its crystal structure (Figure 4), the 31P NMR spectrum of 4 shows two doublets at 66.4 (J = 86 Hz) and 67.0 (J = 85 Hz) ppm suggesting that 4 is dimeric in solution. To further confirm its dimeric structure in solution, pulse gradient spin echo nuclear magnetic resonance (PGSE-NMR) experiments[30-32] were performed (Figure S60) to obtain the hydrodynamic radius of 4 as 7.840 Å, which is comparable to that of 2 (8.144 Å) and distinguishable from that of a monomeric Mo-nitride complex (acriPNP)Mo(N)(CO) (5-CO) (6.242 Å), vide infra. Alternatively, complex 4 can be obtained from the reduction of 3 in 92% yield or the reaction of 2 with NaN3 in 85% yield, see the Supporting Information. While the exact sequence of events leading to chloride extrusion and N2 coordination is unclear, the Liao group reported the formation of an unstable, anionic [(PNCNP)Mo(N)(I)]− species via reduction of the corresponding neutral species.[14] Given the quasi-reversible reduction of 3, the formation of an analogous [(acriPNP)Mo(N)Cl]− species is proposed. Subsequent N2 binding and chloride dissociation leads to the formation of 4. Considering that protonation induces N2 cleavage in A, such protonation is not required for 2 to generate 4.[13] The key difference between the two almost identical Mo complexes is that the central N donor of the rigid acriPNP ligand exhibits reduced Mo–Namide π-bonding, while a flexible PNP ligand with a saturated alkyl backbone significantly contributes to the π-interactions within the Mo2N2 moiety. According to the DFT analysis on 2, the Mo d orbitals mainly establish π-bonding with the bridging N2 π* orbitals, while the HOMO-2 and HOMO-3 reveal mostly a central N(2p) orbital character (Figure S98). This electronic structure aspect influences the reduction potential of 2 and reductive N2 cleavage.
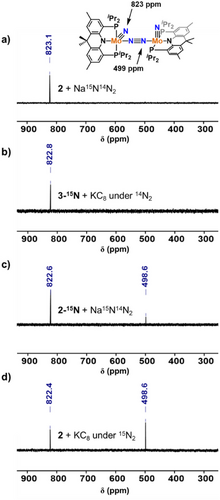
To explore the different pathways for the formation of 4 and provide 15N NMR spectroscopic characterization, 15N labeling studies were performed.[33] Addition of 15N-labeled sodium azide (Na15N14N2) to 2 leads to the formation of the isotopologue 4–15N, in which a single 15N NMR resonance at 823 ppm corresponding to the nitride moiety is observed (Figure 2a). A similar 15N chemical shift at 832 ppm was reported for a related Mo-nitride species.[19] This result likely indicates the formation of a transient Mo-azide species via salt metathesis. The IR spectrum of 4 features an isotopically sensitive Mo≡N stretching band at 1016 cm−1 (Δ(15N) = 28 cm−1). Photolysis of 2–15N2 leads to the formation of 3–15N, in which the nitride moiety is derived via cleavage of the bridging diazenido moiety. As expected, reduction of 3–15N under 14N2 leads of 4–15N, featuring a single 15N resonance for the nitride moiety (Figure 2b). To obtain the 15N NMR resonance for the bridging N2 moiety of 4, 2–15N2 was treated with Na15N14N2. The resulting product 4–15N(15N2) displays a new 15N resonance at 499 ppm, assigned as the bridging N2 moiety of 4 (Figure 2c). However, the intensity of the peak for the N2 moiety was significantly diminished compared to that of the nitride moiety, and we hypothesized that the bridging N2 moiety of 4 could exchange with free N2 in the atmosphere.[34] Finally, reduction of 2 under an atmosphere of 15N2 led to the formation of the fully labeled 4–15N(15N2) species in which the two 15N NMR resonances integrate in a 1:1 ratio (Figure 2d). The Raman spectrum of 4 displays an N≡N stretching band at 1688 cm−1, consistent with a significantly activated bridging N2 moiety (Figure S83). Combined, our synthetic studies led to the isolation of the first example of a metal-nitride-dinitrogen species.
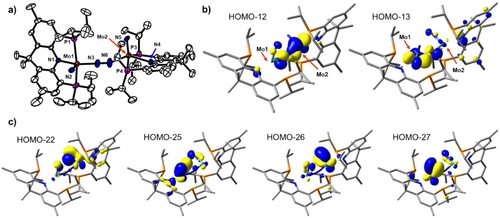
Having access to the bridging N2 complexes 2 and 4, geometric and electronic structures were compared. In 4, the average Mo≡N distance of 1.67 Å is similar to that of 1.652(6) Å in 3 (Table 1). Meanwhile, the N─N distance of 1.183(1) Å in 4 is significantly shorter than that of 1.255(5) Å in 2, indicating that π-backdonation in 4 is significantly reduced due to its higher Mo(IV) formal oxidation state. Considering this description, however, the Mo─N2 and N─N distances in 4 correlate well with other Mo2(µ-N2) complexes as shown in Figure S1 and represent a more activated N2 moiety than some dinuclear Mo(0) µ-N2 complexes.[35] This is further supported by their N─N stretching frequencies (1361 cm−1 for 2 vs. 1688 cm−1 for 4). Interestingly, the orientation of the acriPNP ligands differs between 2 and 4. The two planes defined by the acriPNP fragments are parallel to each other in 2 (Figure 3b), while the corresponding planes are orthogonal in 4 (Figure 4a). The geometry indices of the two complexes are also significantly different, supporting higher contribution of dxz and dyz orbitals of Mo within the metal-nitride moiety of 4 due to its strong triple bond, compared to the reduced electronic effect by the Mo-diazenide π-backbonding interactions in 2. Based on the DFT analysis of 4, each Mo center possesses a doubly filled dxy orbital engaged in π-backbonding interactions between Mo and N2, as shown in Figure 4b, while each Mo ion possesses two orthogonal π-bonding between Mo and N, as shown in Figure 4c. Accordingly, the orthogonal π-backbonding interactions between N2 and two structurally rigidified (acriPNP)MoIV(N) fragments featuring filled dπ orbitals lead to the first example of an N2-bridged Mo(IV, IV) species 4. While the formal oxidation state is assigned as MoIV, 4 behaves like a low-valent Mo species, accepting the weakly-coordinating N2 ligand. The distinct electronic structures of 2 and 4 result in different reactivity of the accommodated N2. While the highly activated N2 moiety in 2 is homolytically cleaved to generate a molybdenum-nitride species, the N2 moiety in 4 is liberated from the molybdenum center and allows the coordination of monodentate ligands, vide infra.
Reductivity of the Mo(IV) Nitride–Dinitrogen Species
The weakly-coordinated dinitrogen moiety of 4 enables substitution reactions to take place with stronger σ-donors and π-acceptors such as PMe3, CO, and CNtBu to give diamagnetic Mo species (acriPNP)Mo(N)(PMe3) (5-PMe3), (acriPNP)Mo(N)(CO) (5-CO), and (acriPNP)Mo(N)(CNtBu) (5-CNtBu) (Figure 5). The 31P NMR spectrum of 5-PMe3 displays a triplet at 9.0 ppm and a doublet at 71.6 ppm with JPP = 9.6 Hz. The 31P NMR spectrum of 5-CO and 5-CNtBu exhibits a singlet at 70.3 and 70.7 ppm, respectively. The single crystal X-ray structures of the three complexes feature five-coordinate, distorted square pyramidal geometries (τ5 = 0.08∼0.18)[36] with an axial nitride ligand (Figure 3d–f). Within error, the Mo-nitride distances are nearly equivalent in the three species, at 1.67 Å (Table 1). The carbonyl and isocyanide stretching bands in 5-CO and 5-CNtBu were observed at 1937 and 2133 cm−1, respectively. Only four other crystallographic examples of Mo-nitride carbonyl/isocyanide species have been reported thus far.[37-39] With the isolation of complex 4 adopting a labile N2 ligand, a large variety of Mo-nitride species featuring a monodentate ligand can be envisioned, and further reactivity studies between the nitride and the bound monodentate species were performed.
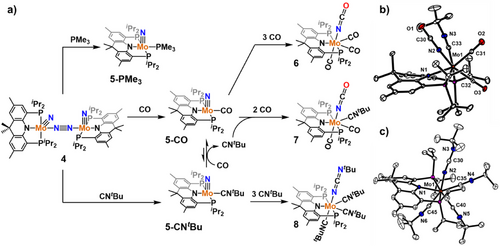
While 5-PMe3 does not react further with excess PMe3, suggesting a nucleophilic nitride character,[40] 5-CO slowly undergoes reaction with CO at 50 °C resulting in the formation of the isocyanate species (acriPNP)Mo(NCO)(CO)3 (6), featuring a broad 31P singlet at 63.2 ppm. The solution IR data shows a peak at 2229 cm−1 along with additional peaks at 2015, 1943 and 1904 cm−1 supporting the presence of NCO and CO moieties. These carbonyl stretching frequencies are comparable to those of an analogous complex (PNCNP)Mo(CO)3I at 2018, 1948 and 1913 cm−1.[41] Furthermore, the Mo≡N stretching frequency at 1016 cm−1 in 5-CO disappears upon carbonylation, which is also indicative of NCO generation (Figure S80). Interestingly, the reactivity of 5-CO with CO(g) can be differentiated from that of 3. While both 5-CO and 3 accommodate a nitride moiety, 3 is inert toward carbonylation under similar conditions. Similar reactivity with related metal-nitride species has been previously observed.[42-47] The Agapie group investigated the distinct reactivity of two Mo-nitride species supported by a p-terphenyl-diphosphine ligand. While the reduced, anionic MoII-nitride enables nucleophilic attack to CO producing a cyanate salt along with a molybdenum carbonyl species, the oxidized MoIV-nitride was inert toward CO.[39] In this regard, our reactivity studies are interesting as high-valent metal-nitrides generally engage in electrophilic reactivity at the π* orbital of the metal–nitride bond.[48, 49] Similarly, the carbonylation of (PNP)WIV(N)(CO) to generate (PNP)W(NCO)(CO)2 was reported by the Schneider group.[50] Due to the difficulties associated with the lability of CO in 6, the analogous isocyanide species was employed to study the reactivity of the MoIV≡N fragment. At room temperature, treatment of 5-CNtBu with an additional 3 equiv. of CNtBu leads to a broad singlet at 61.2 ppm with corresponding 1H NMR and IR data consistent with the formation of the carbodiimide species (acriPNP)Mo(NCNtBu)(CNtBu)3 (8) with loss of the Mo≡N stretching frequency around 1015 cm−1 (Figure S82).[51] Other intermediates such as Mo(N)(CNtBu)2 or Mo(NCNtBu)(CNtBu)2 with varying amount of CNtBu were not observed (Figures S47–S54). These results suggest that the low-valent MoII strongly prefers coordination of three CNtBu ligands to generate the 18 electron species 8 (Figure 5c).
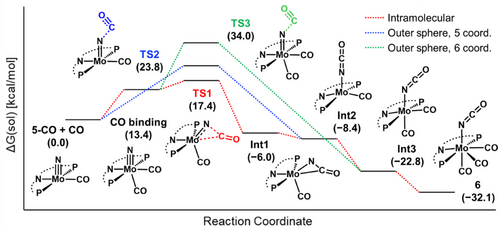
To better understand the mechanism of nitride carbonylation from 5-CO to 6, DFT calculations were carried out. Two reaction mechanisms were considered, the direct nucleophilic attack of nitride to CO and the intramolecular C–N coupling of the coordinated CO. Initially, the nucleophilic attack of the molybdenum-nitride moiety in 5-CO to an empty π* orbital of an outer-sphere CO was considered (Figure 6a, blue pathway). This step has an energy barrier of 24 kcal mol−1 relative to the separated reactants, and the resulting product 6 is exergonic by 32 kcal mol−1. Alternatively, we considered a nucleophilic pathway proceeding upon additional coordination of CO (Figure 6a, green pathway). Initial binding of an additional CO to 5-CO is uphill by 13 kcal mol−1, and subsequent outer-sphere nucleophilic attack of CO has an energy barrier of 21 kcal mol−1, similar to the first, blue pathway. Finally, we tested the inner-sphere pathway proposed by Cleaves and co-workers.[46] They reported the formation of cyanate from carbonylation of a uranium-nitride species. Computational studies suggest that the reaction proceeds via CO coordination and formation of a metallacyclic transition state. Upon initial binding of CO to 5-CO as discussed above, we find a low energy transition state of only 4 kcal mol−1 above in energy for the intramolecular, nitride migratory insertion pathway (Figure 6a, red pathway). This leads to a side-on isocyanate species (acriPNP)Mo(η2-NCO)(CO), intermediate 1. Subsequent CO binding leads to the formation of downstream intermediates 2 and 3, which are shared between the two outer-sphere pathways. The HOMO-2 of the lowest energy intramolecular transition state, TS1, features mixing of the Mo≡N π bonding orbital with the π* orbital of CO (Figure S114). In short, computational studies favor an associative nitride migratory insertion pathway over an outer-sphere nucleophilic pathway.[52, 53]
To provide experimental evidence for the DFT calculated mechanism, we attempted the carbonylation of 5-CO in the presence of 1 equiv. of CNtBu, with the hypothesis that the availability of the strongly binding isocyanide species in solution would facilitate access to the six coordinate (acriPNP)Mo(N)(CO)(CNtBu) intermediate and accelerate nitride migration. Indeed, while the conversion of 5-CO to 6 requires mild heating at 50 °C for complete conversion in 40 h, formation of the mixed species (acriPNP)Mo(NCO)(CNtBu)(CO)2 (7) proceeds at room temperature during the same time (Figure 5). Alternatively, 5-CNtBu can be carbonylated at room temperature, leading to the rapid formation of 5-CO via ligand exchange and subsequent conversion to 7. While 6 readily loses CO to form the bis-carbonyl species, the isocyanide bis-carbonyl species 7 is inert toward CO loss and was crystallographically characterized (Figure 5b). While formation of 7 may proceed via ligand exchange of 6-CO with CNtBu, this would not explain the observed increase in the rate of NCO formation.
Conclusion
In summary, we present a comprehensive structural, spectroscopic, and computational study of reductive N2 cleavage at a (acriPNP)Mo scaffold, leading to the isolation of a bridging diazenido species and a unique dimolybdenum species (4) possessing both nitride and N2 coordination. Complex 4 is the first example of a Mo(IV) nitride–dinitrogen species, featuring two orthogonal π-backbonding interactions between N2 and two structurally rigidified MoIV(N) fragments. Complex 4 displays low-valent like reactivity, leading to the isolation of phosphine, CO, and isocyanide adducts. Further reactivity with CO leads to nitride carbonylation to isocyanate via an intramolecular, associative nitride insertion pathway. Our work inspires further studies in direct N2 fixation to value-added products via nitride functionalization with π-acidic substrates.
Supporting Information
The authors have cited additional references within the Supporting Information.[54-72]
Acknowledgements
This research was supported by the National Research Foundation of Korea (2022M3C1A3092056 to Y.L, RS-2023–00240996 to J.C.). The authors thank Dr. Dongwook Kim at the Institute for Basic Science for discussions on XRD data analysis.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




