Synthesis and Structure of Neopentyl Sodium: A Hydrocarbon-Soluble Reagent for Controlled Sodiation of Non-Activated Substrates
Dedicated to the memory of Dr Ivan Lavandera
Abstract
While recent studies have shown that organosodium reagents can offer excellent promise in organic synthesis, their widespread applications can be hindered by their limited solubility in organic solvents, lack of stability, and uncontrolled reactivity. Improving this immature area, here we report the structure of unsolvated neopentyl sodium, which exhibits a unique discrete tetrameric motif and good solubility in hexane. Combining spectroscopic and crystallographic studies with computational bonding analysis, key insights into its constitution and stability have been gained. Expanding its synthetic potential, supporting neopentyl sodium with Lewis donor PMDETA (N,N,N′,N″,N″-pentamethyldiethylenetriamine) allows for the direct metalation of challenging non-activated arenes and alkenes, including the regioselective meta-functionalisation of selected 1,3-disubstituted alkyl arenes. Shedding light on sodium-mediated metalation protocols, key organometallic intermediates have been isolated and structurally defined, which can subsequently be intercepted with CO2 or a boron electrophile to be directly used in Pd-catalysed Suzuki cross coupling reactions.
Introduction
Polar organolithium reagents constitute one of the most widely used class of organometallic reagents employed for functionalisation of organic molecules.[1-4] Much of their success has come from having an in depth understanding of the constitutions of key organometallic intermediates leading to their rational utilisation.[5-9] However, despite the common usage of organolithiums, organosodium reagents have started to appear in synthetic endeavours, driven by attempts to replace scarcer and more expensive lithium with the more abundant and sustainable sodium metal.[10-15] Promisingly, these studies have revealed that some sodium organometallics can also exhibit superior but controllable reactivities.[14-16]
Interestingly, organosodium compounds have been known for over 150 years since Wanklyn's 1858 report of ethyl sodium via reaction of ethyl iodide with sodium metal.[17] While organosodium chemistry was neglected for many years, seminal work from Morton and Finnegan in the 1950–60s hinted at their potential in metalation chemistry using n-pentyl sodium for the sodiation of non-activated substrates (pKa values > 40) such as benzene and assorted alkenes.[18-25] Here, the alkyl sodium was accessed via n-pentyl chloride and sodium metal, affording a reportedly insoluble mixture of sodium chloride and the organosodium reagent. In general, the sensitive nature, poor solubility, and limited solvent tolerance of in situ sodiated species (intermediates) meant they could not be isolated in pure form or characterised, with these reactions only monitored via CO2 electrophilic interception, producing moderate to good yields of the relevant carboxylic acids (15%–69% yields) with reaction times ranging from 1 h to several days.
Considering the operational challenges of the preparation of alkyl sodium reagents, their use in organic synthesis has continued to be very limited until recently, where new methods have been developed using alternative sodium sources. Knochel has shown that the soluble but thermally unstable 2-ethylhexyl sodium can be made by passing 2-ethylhexyl chloride through a sodium packed column under continuous flow conditions.[26] This in situ formed alkyl sodium was found to be a powerful base capable of sodiating a variety of toluene derivatives for further functionalisation, as well as being a competent reagent for sodium halogen exchange applications.[27]
It has been reported that combining finely divided sodium in dispersion or brick form with neopentyl chloride produces a potent but surprisingly soluble alkyl sodium base which can be used in sodium/bromine exchange reactions with a wide range of bromoarenes. This has been demonstrated by Takai and Asako, where the generated NaAr intermediates can be used in Pd- catalysed cross couplings (Scheme 1a);[28-30] and by Capriati who reported such NaAr species react with a range of electrophiles using water or deep eutectic solvents (DESs) under air.[31] Remarkably, while neopentyl sodium (NaNp) seems to be key in these studies, its constitution or aggregation has not been pursued (Scheme 2).
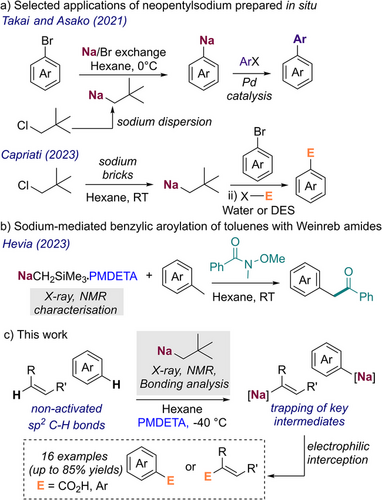
Using a different method to access alkyl sodium reagents, our group has recently reported the use of NaCH2SiMe3, isolated as a pure reagent from the reaction of LiCH2SiMe3 and NaOtBu for the benzylic aroylation of toluenes using Weinreb amides (Scheme 1b).[16, 32] Through the successful isolation of key organometallic intermediates, this study elucidated the key role of Lewis donor PMDETA to promote these transformations, enhancing the kinetic reactivity of these intermediates towards both metalation and nucleophilic addition. Related to this work, Lu has also shown the ability of NaCH2SiMe3 to promote methylenations of ketones and aldehydes.[14] Furthermore, early this year, Thomas has reported that pyridine can be regioselectively C4-functionalised by direct metalation with n-butylsodium under carefully controlled reaction conditions.[33]
Putting alkylsodium chemistry on a more practicable basis, here we isolate and fully characterise solvent-free NaNp prepared by simple salt metathesis of LiNp with NaOtBu. Combining X-ray crystallographic and spectroscopic analysis with bonding analysis calculations[34] (QTAIM,[35] NCI[36] and ELI-D[37]), we shed light on the constitution, solubility, and aggregation of this reagent. Its ability to mediate direct Na-H exchange reactions is also demonstrated by probing its reactivity towards a wide range of non-activated arenes and alkenes, disclosing a superior metalating power to those observed by conventional organolithium reagents or even by Lochmann–Schlosser super basic combinations.
Results and Discussion
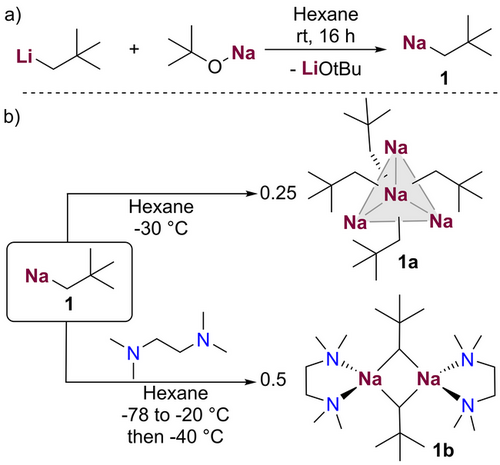
Building on our previous studies,[38] NaNp (1) was prepared by salt-metathesis by treating neopentyl lithium with sodium tert-butoxide in hexane which led to the isolation 1 as a white solid in a 53% yield (see Supporting Information for details). Note that this moderate isolated yield is due to the high hexane solubility of 1, which is remarkable since it contrasts markedly with the poor solubility of other alkyl sodium compounds such as NanBu or NaCH2SiMe3 also prepared using this method.[38, 39] In fact, we found that 1 can be stored as a 0.5 M solution in hexane for at least 3 days at 20 °C without observing decomposition. This unique solubility also allowed us to fully characterise 1 in deuterated cyclohexane solutions by NMR spectroscopy. Its 1H NMR spectrum showed a distinct signal at −0.71 ppm assignable to the Na-CH2 group, which is slightly more shielded than the analogous signal in congeneric LiNp (−0.61 ppm).
When forming a solution of a mixture of 1 in the presence of LiOtBu, a crop of colourless needle-like crystals of [{NaCH2tBu}4] (1a) were formed. In addition, we found that upon cooling a solution of pure neopentyl sodium in hexane to −30 °C, the same crystalline product was attained. X-ray crystallographic studies of 1a revealed a discrete tetrameric structure (Figure 1a ii) which can be described as a distorted tetrahedron of four sodium atoms, with each triangular Na3 face capped by a methanide carbon atom to complete a distorted Na4C4 heterocubane. While this tetrameric motif bears a resemblance to that exhibited by the classical organometallic reagent tBuLi,[40] its discrete motif is unusual when compared with the structures of other unsolvated alkyl sodium complexes.[41-43]
A close inspection of the geometrical parameters in 1a show that the Na─C bond distances (range 2.6324(21) to 2.6733(20) Å)] are within the same ballpark as those previously reported for related alkyl sodium complexes.[32, 41, 44] The non-bonding Na…Na distances in 1a (spanning from 3.0500(11) to 3.2244(10) Å) compare well with those found for unsolvated, silyl-substituted [{NaCH2SiMe3}4]∞,[41] which also comprises {Na4C4} tetramers, although, in this case, these units interact intermolecularly with each other in a supramolecular arrangement.[43] Thus, in 1a the shortest Na─C atomic distance between two different oligomeric units is 3.5516(20) Å; whereas in [{NaCH2SiMe3}4]∞ it contracts to 2.7872(17) Å. This discrete tetrameric structure of 1a is retained in deuterated cyclohexane solutions, as evidenced by 1H DOSY NMR studies (estimated FW = 358 g mol−1, 4% error, see Supporting Information for details),[45, 46] which explains its remarkable hydrocarbon solubility.
Solvated adducts of 1a could be formed by adding equimolar amounts of TMEDA (N,N,N′,N′-tetramethylethylenediamine) or PMDETA to solutions of NaNp in hexane. These proved highly reactive decomposing above −40 °C (see Supporting Information for details). While the PMDETA adduct decomposed on attempts to isolate it, the TMEDA adduct was more successful, which could be isolated at −40 °C as colourless crystals, that were amenable to X-ray crystallographic characterisation. Thus, [{TMEDA)NaCH2tBu}2] (1b) (Figure 1b ii) adopts a centrosymmetric dimeric motif, containing a nearly planar four-membered (NaC)2 ring (sum of internal angles, 358°) where the neopentyl groups bridge the Na atoms. The Na1─C1 bond is noticeably shorter to those found in 1a [2.359(9) versus a mean value of 2.6528 Å] which can be rationalised in terms of the different coordination number of the CH2 unit in both compounds. Interestingly the remaining Na─C bond distance in 1b is significantly elongated [Na1─C1’ 2.823(9) Å] and both neopentyl groups lie on the same side of the ring, although there is not an obvious explanation to account for this preferred cisoid conformation.[47] This dimeric structure contrasts with that reported for the related TMEDA adduct of NaCH2SiMe3 which forms a polymeric chain arrangement,[41] indicating that the steric demands of the neopentyl group seem to favour formation of smaller aggregates. The dimeric aggregation 1b appears to be retained in deuterated cyclohexane solutions as indicated by 1H DOSY NMR experiments (estimated weight of 377 g mol−1, 11% error, see Supporting Information for details.).[45, 46]
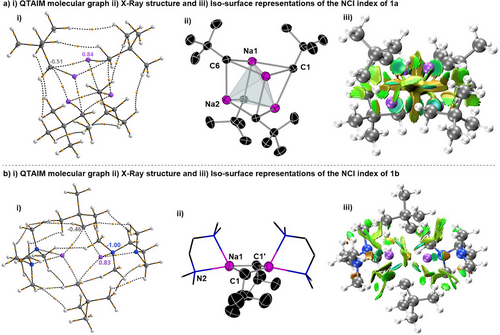
Intrigued by the structural features of 1a and 1b we next sought to further understand the nature of the bonding of the complexes with the aid of computational techniques, using a combination of QTAIM and NCI [Figure 1i and iii] and electron localisability indicator (ELI-D) analyses (Figure 2). Remarkably, for both compounds it was deduced that inclusion of the empirical dispersion correction in the isolated-molecule geometry optimisation (B3LYP/def2-TZVP) leads to a significant total stabilisation of the molecular aggregates by 443.8 and 401.3 kJ mol−1 for 1a and 1b, respectively. This is caused by conformational rearrangements in the methyl groups of both the neopentyl and TMEDA groups (for 1b) upon activating dispersion correction (see also Figure S66), so that the ligands form a kind of organic skin around the Na atoms stabilised by London dispersion.
The Me–Me attractive dispersion interactions can be visualised as bond paths between hydrogen atoms in the QTAIM molecular graphs (Figure 1 i), which are channels of interaction between atoms in the electron density.[48] While for 1a two H─H bond paths between each pair of adjacent Np groups has been found, it should be noted that these H─H bond paths are absent in its electron-density distribution when empirical dispersion correction is not included (Figure S66 in the Supporting Information). NCI analysis provides an alternative visualisation of these van-der-Waals-type interactions (Figure 1 iii), displaying attractive dispersion forces between the methyl groups of the Np ligands in both complexes around the central sodium core (green colour patches in Figure 1 iii). Collectively, these findings emphasise the profound effect of dispersion to rearrange the conformation of the alkyl groups, providing significant stabilisation by their mutual interactions to favour the formation of these unusually low-aggregated organosodium complexes.[49-51]
In contrast to the geometrical parameters obtained by the X-ray crystallographic studies, the isolated-molecule optimised geometries are much more symmetrical concerning the central Na cluster (optimised Na─C bond distances in the caption of Figure 1) as expected when the crystal field of discrete neighbours is lost. The Na─C distances also become more similar between both compounds (2.54–2.58 Å) upon symmetrisation. Consistent with the discussion of the experimental geometries, for 1a each of the CH2 groups of the Np ligands is located centrally above one of the Na3 triangles from the faces of the Na4 tetrahedron, bridging those three Na atoms with three separate bond paths, to form a distorted cubic structure (Figure 1a,i). Similarly, for 1b, each CH2 lies close to the Na…Na axis, to form a NaCNa bridge indicated by two bond paths to the two Na atoms (Figure 1b,i).
The delocalisation index (DI) that measures the exchange of electron pairs between two atomic basins in QTAIM; can be considered as a bond order.[52] It is independent of the existence of bond paths, so it can measure the interaction of the Na atoms with each other. However, none of the Na–Na DI's in 1a and 1b exceeds a value of 0.005, indicating that their mutual interaction can be considered negligible. In contrast, the DI values for the C–Na interactions are significant although still low. Thus, in 1b, the DI sum for each bifurcated C–Na2 interaction is 0.24, whereas in 1a, a value of 0.30 was found for the C–Na3 units. These findings indicate that the covalent contributions to these bonding interactions are low, which can be rationalised by the fact that the carbon atoms cannot become hypervalent although they are penta- or hexa-coordinated. Furthermore, the NCI analysis of the complexes shows that there are significant areas of repulsion in the central spaces between the Na atoms (Figure 1 iii).
Atomic charges for 1a and 1b were calculated by QTAIM (Figure 1,i), finding that despite their different aggregation, almost identical values were found for the Na atoms (0.84 and 0.83 e, respectively). For the CH2 units of the Np ligands values close to half a negative charge were found (−0.51 and −0.46 e for 1a and 1b respectively), leaving an overall positive charge which is counterbalanced in 1a by the methyl groups which are slightly negatively charged. In 1b, the TMEDA nitrogen atoms overcompensate this residual charge of the Na/C core with −1.00 e per N atom, which in turn means that the methyl groups are each slightly positively charged.
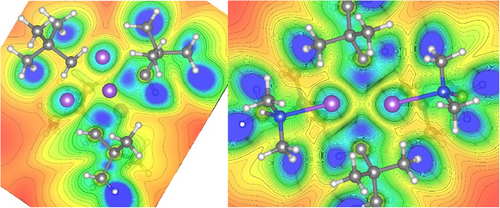
ELI-D distributions analysis was also performed (Figure 2 and see Supporting Information for details) which shows high localisation of the electron pairs on the CH2 units of the Np groups and also how these pairs are directed within 1a and 1b. For 1a the basins of these electron pairs share boundaries in topological analysis with three Na atoms and their parent C (Figure 2, left); whereas for 1b they are shared with one C and only two sodium atoms (Figure 2, right). These findings are consistent with the description of the bonding present in 1a and 1b as [4c, 2e-] and [3c,2e-] respectively.
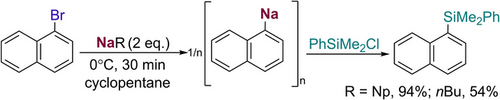
Turning back to our experimental studies, we pondered if the exceptional hydrocarbon solubility of 1 could be leveraged to access reactivity that would be otherwise unattainable for an insoluble alkyl sodium such as NanBu.[38] Thus we first tested the reactivity of isolated donor free NaNp towards Na/Br exchange using 1-bromonaphthalene as a model substrate. Using cyclopentane as a solvent, under mild reaction conditions (0 °C, 30 min), rapid formation of 1-napthylsodium occurs which can be intercepted with PhSiMe2Cl to form dimethyl(naphthalen-1-yl)(phenyl)silane in a 94% yield (see Scheme 3 and Supporting Information for details). This result aligns well with previous studies by Asako and Takai on the use of NaNp, prepared in situ using Na dispersions and NpCl (Scheme 1a).[29] Contrastingly using NanBu, under exactly the same reaction conditions, furnished the relevant silane product in a diminished 54% yield (Scheme 3).
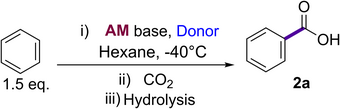
We next investigated the reactivity of NaNp towards C─H metalation, an area in which other organosodium reagents have also shown promise. We focused on non-activated arenes and alkenes (in terms of pKa values) which are challenging to metalate with conventional s-block metal bases, using benzene (calculated pKa about 44)[53] as a model substrate. Disappointingly, addition of 1.5 equivalents of benzene to 1a in hexane at room temperature, followed by interception with CO2 furnished only traces of carboxylic acid 2a (Table 1, entry 1). However, addition of TMEDA to generate 1b in situ led to the formation of 2a in a 62% yield (Table 1, entry 2), establishing the dramatic effect a donor has on this reaction, favouring the formation of a less aggregated and consequentially more kinetically active organosodium base. Importantly, the reaction needed to be carried out at −40 °C to avoid competing metalation of TMEDA. Increasing the denticity of the Lewis donor by using PMDETA furnished 2a in a high yield (83%) (Table 1, entry 3), whereas the use of ethereal solvents as donors was detrimental to the overall reactivity.
 |
|||
| Entry | AM Base | Donor | Yield (%) |
|---|---|---|---|
| 1 | NaNpa) | None | traces |
| 2 | NaNp | TMEDA | 62 |
| 3 | NaNp | PMDETA | 83 |
| 4 | LiNp | PMDETA | 14 |
| 5 | NaCH2SiMe3 | PMDETA | 21 |
| 6 | NaTMP | PMDETA | traces |
| 7 | LiCKOR | PMDETA | 56b) |
| 8 | LiNp + NaOtBu | PMDETA | 49 |
| 9 | KNpc) | PMDETA | 79(49)d) |
- Conditions: i) alkali metal base (1 mmol), Lewis base donor (1 mmol), benzene (1.5 mmol), hexane (5 mL), −40 °C, 4 h; ii) CO2 (1 atm); iii) hydrolysis with 1 M HCl. Yields determined by 1H NMR using internal standard of hexamethyl benzene (10 mol%).
- a) reaction carried out at room temperature;
- b) several products observed by 1H NMR.
- c) See Supporting Information for details.
- d) Reaction performed in the absence of PMDETA.
Interestingly, when comparing these optimised reaction conditions to other organometallic bases we found that in the presence of PMDETA the NaNp was remarkably superior to the congeneric lithium reagent LiNp which yielded 2a in a poor 14% yield (Table 1, entry 4), this lowered Brønsted basicity of the alkyl lithium presumably arising from the less polar nature of the Li─C bond compared to the Na─C bond of alkyl sodiums. The identity of the alkyl group was also found to be important, as when the powerful alkyl sodium NaCH2SiMe3[34] was used 2a was obtained in a modest 21% yield (Table 1, entry 5). Sodium amide NaTMP (TMP = 2,2′,6,6′-tetramethylpiperidide) also failed to metalate benzene under the conditions of the study, (Table 1, entry 6).[54] Moreover, the classical Lochmann–Schlosser LICKOR superbase in the presence of PMDETA proved better but the yield was again diminished (56%; Table 1, entry 7) compared to our new base combination and no product was observed in hexane with the absence of PMDETA. Incomplete superbase metalations have previously been attributed to some side reactions such as multiple metalations on the benzene ring.[55] Combining LiNp with NaOtBu in situ also resulted in a lower yield of 2a (49%) (Table 1, entry 8), thus highlighting the superior behaviour of the pure isolated alkyl sodium reagent for a more effective performance of the Na─H exchange process. Interestingly it should be noted that addition of LiOtBu to a solution of NaNp/TMEDA in hexane did not have an inhibitory effect on the metalation of benzene. Finally, the potassium analogue (KNp) was tested (Table 1, entry 9) which in the presence of PMDETA formed 2a in comparable yields (79%) to those observed for NaNp. Notably, in the absence of PMDETA the yield of 2a decreased to 49%.
Building on these findings, we next investigated the scope of our metalations to other non-activated and alkyl-substituted arenes (Scheme 4).
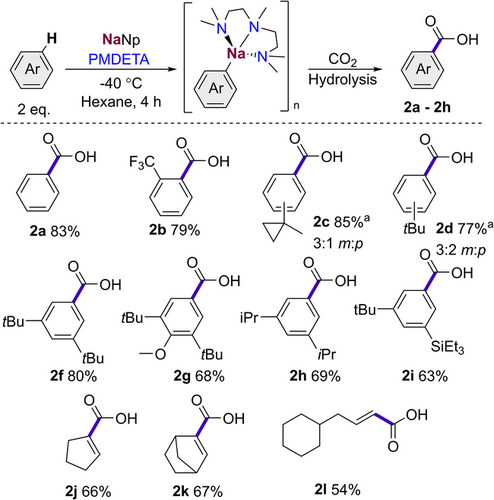
The presence of an acidifying trifluoromethyl group led to the formation of ortho-substituted 2b in a good yield of 79%, this result is in agreement with our previous studies where the metalation of this substrate at −78 °C with n-butyl sodium was investigated.[56] When introducing a bulky alkyl group onto the aryl ring without an available benzylic proton, such as in 1-methylcyclopropyl or tert-butyl, we found facile metalation under our conditions to form the corresponding carboxylic acids 2c and 2d after a CO2 quench and subsequent hydrolysis in excellent yields (85% and 77%). Interestingly, these bulky groups show a remote directing effect favouring the formation of a mixture of the meta and para metalation (in a 3:1 m:p ratio or 3:2 m:p ratio for 2c and 2d respectively). The good yields for 2c and 2d contrast with those reported by Schlosser using the LiCKOR superbase, showing these substrates could be carboxylated only in modest yields of 53% and 52%, respectively,[55] whereas a low yield of 15% was achieved when using pentyl sodium for the metalation of tert-butyl benzene at room temperature for 4 h.[57] On the basis of NMR studies, here the authors tentatively propose that the electron rich alkyl groups significantly slow the metalation on the aryl rings due to a σ- coupled deformation of the π- electrons in the rings.[58] Thus, the high yields observed for these substrates when using neopentyl sodium as a base solvated by PMDETA further exemplifies the power of this highly basic combination.
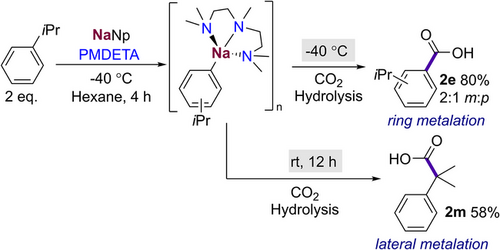
We found that introducing a substrate with a poorly accessible benzylic proton such as in isopropylbenzene gave solely aryl metalation, affording 2e as a mixture of meta and para isomers in a good yield of 80% after 4 h at −40 °C (Scheme 5). However, when the reaction was allowed to warm to room temperature and left to stir overnight, there was full conversion of the aryl sodium to the benzylic species, which can be then intercepted with CO2 to give lateral metalation product 2m in a 58% yield, showing the ability of the anion to migrate over time to the more stabilised position. These findings concur with previous studies by Crimmins, where addition of TMEDA and isopropylbenzene to a pentylsodium suspension in hexane resulted in the selective lateral metalation over a period of 24 h.[59]
Next, we sought to introduce multiple bulky functional groups onto our substrates to achieve more selective functionalisation. In this regard Schlosser has shown that LiTMP can direct the remote meta-metalation of (2,6-dichloro-phenyl)triethylsilane.[60] More recently Knochel has elegantly demonstrated the ability of 1,3-bis(triethylsilyl)-substituted arenes to undergo selective lithiation at their C5 position.[61] Furthermore, Antonov has also shown, with the aid of computational studies, the effective utilisation of non-covalent interactions for switching the regioselectivity of the lithiation of pyridine derivatives.[62] We hypothesised that the incorporation of two bulky substituents meta to each should direct the selectivity on the metalation process due to the increase of steric congestion. We were pleased to find that 1,3-ditertbutylbenzene is metalated in the 5- position to afford 2f in an excellent yield of 80%. This approach was then extended to 2,6-di-tert-butyl-anisole, 1,3-diisopropylbenzene and 3-tertbutylphenyltriethylsilane giving the relevant meta-substituted carboxylic acids 2 g–2i in yields ranging from 63% to 69% (Scheme 4).
Early studies in the 1960′s have hinted at the potential of alkyl sodium bases to deprotonate cyclic olefins, although harsh conditions with long reaction times are required.[63] With our optimised metalating conditions, we hoped to extend our scope of sodiated species from benzene derivatives to cyclic and more strained alkenes. Starting from the more acidic cyclopentene, we were pleased to observe excellent metalation and subsequent carboxylation under our standard conditions to give 2j in a reasonable yield of 66%, with no sign of competing allylic metalation. However, adding one more carbon to the ring in the form of cyclohexene, led solely to allylic metalation. Unfortunately, we found a mixture of products formed in the CO2 quench, so a phenyl Weinreb amide quench was used, giving the corresponding ketone product 4ba in a reasonable yield (58%) (see Supporting Information). Finally, we found that adding more strain to the cyclohexene ring in the form of norbornene helped to direct the metalation to the alkene, which could be quenched with CO2 to form 2k in a good yield of 67%. Turning our attention to the less activated linear alkenes, metalation and subsequent functionalisation of allylcyclohexane can be accomplished furnishing 4-cyclohexylbut-2-enoic acid (2l) in a reasonable yield of 54%.
We next looked at the challenge of isolating and characterising possible sodiated intermediates from these reactions. Treating tert-butylbenzene and 2,6-di-tert-butyl-anisole in hexane with equimolar amounts of NaNp and PMDETA at low temperature facilitated isolation of [{(PMDETA)Na(3-tBuPh)}2] (3a) and [{(PMDETA)Na(1-OMe-2,3-tBu2-C6H2)}2] (3b) as crystalline solids (see Supporting Information for details). X-ray crystallographic studies established their molecular structures. Both adopt similar dimeric motifs, having a central four-membered (NaC)2 ring, with aryl bridges between the Na atoms, which are further stabilised by binding to PMDETA (Figure 3). It should be noted that while made in situ in solution the metalation of tert-butyl benzene formed a mixture of meta and para metalation, both aryl groups present in solid 3a are meta-metalated. They share the same structural motif as [{(PMDETA)NaPh}2] which was reported by Weiss and made via salt-metathesis of PhLi with NaOtBu.[64]
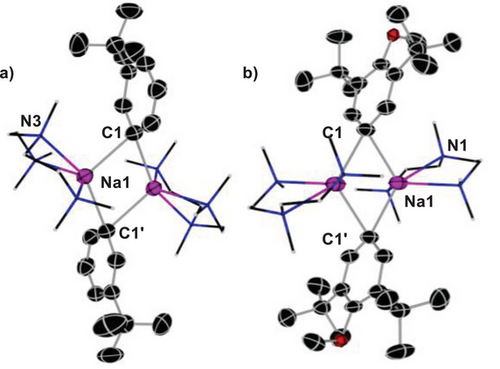
The Na─C bond distances in 3a and 3b [ranging from 2.6255(19) to 2.6659(13) Å] lie within the typical range of those previously reported for other aryl sodium complexes.[46, 56, 65] Compounds 3a and 3b are not stable for long enough in deuterated benzene solutions to acquire meaningful DOSY NMR spectra, though for 3b a diagnostic downfield signal at 196.5 ppm is observable in the 13C NMR spectrum for the NaCAr.
Using a similar synthetic approach to that described for 3a and 3b, [{(PMDETA)Na(2-norbornenyl)}2] (4a) and [{(PMDETA)Na(Cyclohexenyl)}2] (4b) were isolated as crystalline organometallic intermediates (prior to any onward bond-forming reaction) resulting from sodiation of norbornene and cyclohexene in 48% and 44% yields, respectively (Scheme 6a). X-ray crystallographic studies established 4a exhibits a similar dimeric motif to those described for 3a and 3b, with the norbornenyl group bridging between the Na atoms via the C of the C─H bond that has been metalated [Na1─C1 = 2.6029(12) Å, Na1─C1’ = 2.6487(11) Å] (Scheme 6b). The Na atoms also engage in an additional, secondary electrostatic π-interaction with the remaining vinylic CH unit [Na1─C2 = 2.9752(19) Å]. The vinylic character of 4a is also apparent by the length of its C1─C2 bond [1.3054(23) Å] which is consistent with a localised C═C bond. As far as we can ascertain from a search of the Cambridge Structural Database, 4a is the first example of a C2-metalated norbornene complex to be structurally defined. Furthermore, it should also be noted its rare sodium vinylic constitution, for which there are hardly any precedents in the literature.[66]
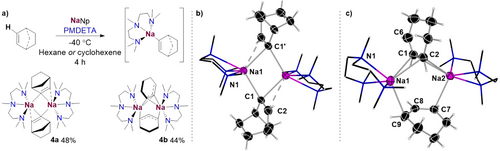
In contrast, the 4b dimer contains two cyclohexenyl bridges, both deprotonated at their allylic position, that coordinate in a non-symmetric fashion to both {Na(PMDETA)} cations and adopt a perpendicular disposition with respect to each other (Scheme 6c).Thus, one allyl group binds in a η2-fashion to both Na atoms [Na1–C1/C2 average distance, 2.7416 Å; Na2–C1/C2 average distance, 2.8793 Å]. The second cyclohexenyl binds to each sodium atom using only one of its terminal allylic carbons [Na1–C9, 2.6823(18) Å; Na2–C7, 2.6509(14) Å]. These values are in accordance with previously reported Na─Callyl bond lengths.[67-69] The allylic nature of the cyclohexenyl fragments was observed by examining the shortened average C─C bond lengths [C2–C1/C1–C6, 1.3851 Å], [C7–C8/C8–C9, 1.3821 Å]. The coordination of the cyclohexenyl groups in 4c is significantly different to those recently reported by us for other PMDETA-solvated sodium allyl complexes containing non-cyclic allyl ligands which form monomeric motifs with the allyl group adopting η3-coordination.[67] A possible rationale for this unusual coordination preference can be the large steric constraints imposed by the cyclic structure of the cyclohexenyl groups (see Figure S68 in Supporting Information for a space filling model representation). As a consequence of this preferred structural arrangement the two Na atoms are significantly further apart from each other than those in 4a [4.4930(6) versus 3.2117(7) Å].
Attempts to assess aggregation of 4a and 4b in deuterated cyclohexane were also unfruitful due to their limited stability in solution which also preluded the acquisition of their 13C NMR spectra (see Supporting Information for details).
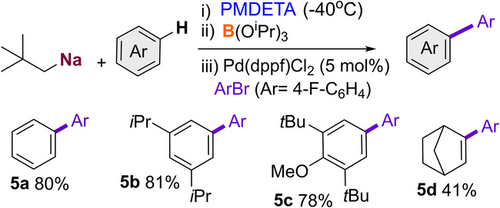
Our previous studies have shown that aryl sodium boronates can be excellent precursors in Suzuki–Miyaura couplings.[54] Using benzene, 2,6-di-tert-butyl-anisole, 1,3-diisopropylbenzene and norbornene as model substates we were delighted to find that the metalation of these substrates with NaNp/PMDETA can be coupled in a sequential one-pot approach with a transborylation step using B(OiPr)3 and a palladium catalysed Suzuki–Miyaura coupling using 1-bromo-4-fluorobenzene and 5 mol% of Pd(dppf)Cl2, to furnish 5a-5d (Scheme 7). Expanding further the synthetic potential of these sodium-mediated transformations, bis(aryls) 5a-c were obtained in excellent yields, ranging from 78% to 81%; though in the case of cyclic alkene norbornene, 5d, the yield dropped to 41%.
Conclusions
Using a salt-metathesis approach we have isolated and characterised solvent-free hydrocarbon soluble neopentyl sodium, a reagent that has shown hints of promise in polar organometallic chemistry but whose constitution has remained elusive for decades. Combining spectroscopic and crystallographic studies with computational studies, valuable insight into the nature of bonding present in this compound and its stability have been gained. Unlike related alkylsodium complexes, NaNp exhibits a rare discrete tetrameric structure that displays high solubility in hydrocarbon solvents, a benefit likely to open up its utilisation in synthetic organic chemistry.
As an example towards such utilisation, here when we combined NaNp with PMDETA remote metalation of non-activated C(sp2) bonds in aryl and olefin substrates was achieved, whereas these substrates cannot be metalated by conventional s-block metal bases such as lithium amides or alkyllithiums. This work could also stimulate development of more organometallic reagents of this environmentally benign, earth abundant metal.
Acknowledgements
The authors thank the University of Bern and the Swiss National Science Foundation (SNSF) (project number 219318) for its generous sponsorship. The Synergy diffractometer used for X-ray single crystal structure determination was partially funded by the SNSF within the R'Equip programme (project number 206021_177033).[70] The authors also thank Robert E. Mulvey (University of Strathclyde) for useful discussions on this research.
Open access publishing facilitated by Universitat Bern, as part of the Wiley - Universitat Bern agreement via the Consortium Of Swiss Academic Libraries.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.




