Light-Driven Ratchet Mechanism Accelerates Regioselective Metal-Cation Exchange in a Heterobimetallic Helicate
Abstract
Molecular machines rely on their capacity to exploit non-equilibrium processes to perform work. However, the development of these non-equilibrium processes, such as molecular ratchets, is still in its early stages. Here, we report a diazocine-containing ligand (L) harbouring two distinct chelating coordination sites that can self-sort into dinuclear homo- and heterobimetallic helicates ([FeII2L](OTf)4, [CoII2L](OTf)4, [ZnII2L](OTf)4, [ZnIIFeIIL](OTf)4, [ZnIICoIIL](OTf)4) with precisely controlled metal cation distribution. The photoisomerisation of the helicates operates via a molecular ratchet mechanism, resulting in metastable diastereomers that shift the system from thermodynamic equilibrium. Continuous white-light irradiation autonomously drives this ratchet process, selectively enriching an out-of-equilibrium pseudo-mesocate structure. Crucially, the ratchet mechanism can significantly accelerate metal-cation exchange from the [ZnII2L](OTf)4 helicate to the [ZnIIFeIIL](OTf)4 helicate. Thus, the system operates in a manner reminiscent of a “claw machine”, selectively seizing FeII ions when subjected to a precisely controllable external stimulus. These findings lay the foundation for creating adaptive and reconfigurable supramolecular structures that use non-equilibrium phenomena on a molecular level.
Introduction
Harnessing endergonic reactions is crucial for operating molecular machinery and creating adaptive materials.[1] A prime example of harnessing endergonic reactions is photosynthesis, which captures light energy to create a proton gradient.[2] This gradient powers ATP synthase,[3] converting ADP into ATP through an endergonic reaction. In essence, photosynthesis acts as a molecular ratchet,[1, 4] coupling an exergonic proton transport process with an endergonic reaction within a multi-step chemical reaction cycle.
Molecular ratchets function unidirectionally and bypass microscopic reversibility,[5] allowing them to access out-of-equilibrium states. Chemical stimuli or light can activate molecular ratchets,[6, 7] with light being a notably appealing option due to its unique benefits: light generates no chemical waste and allows for precise spatial and temporal control.[8] To effectively harness light in artificial molecular ratchets, precisely aligning the photoresponsive building blocks, such as azobenzenes,[9-13] diazocines,[14-16] crowded alkenes[17, 18] or dithienylethanes (DTE),[19, 20] can enhance the switching effect. A key method for organising several photoresponsive building blocks within one structure is through the self-assembly of supramolecular organic or metal-organic capsules.[8, 21, 22] Recently, Feringa, Kathan and colleagues[11] reported a supramolecular organic capsule that functions as a molecular ratchet, using photoisomerisation and imine exchange to form a kinetically trapped, out-of-equilibrium open capsule. In another study, Clever, Herges and co-workers[14] introduced a diazocine-containing ligand that preferentially formed an out-of-equilibrium PdII2L4 lantern when exposed to light in the presence of PdII cations and disassembled in darkness.
Improving the structural complexity of metallo-supramolecular capsules by integrating various ligands or metal cations into heteroleptic[23, 24] or heterometallic[25] structures, or by incorporating asymmetric ligands,[26-28] may broaden their utility in guest binding and supramolecular catalysis, thus achieving the molecular recognition and catalytic regulation akin to that seen in biological host–guest systems.[29] Heterometallic structures are known for their improved magnetic[30] and photophysical properties,[31] along with enhanced redox[32] and catalytic activity[33] compared to their homometallic counterparts. However, accurately self-sorting various metal cations and ligands into heterobimetallic structures presents a substantial challenge, particularly when using metal cations that prefer similar ligand environments. A stepwise synthesis is commonly used,[34-43] starting with the assembly of one kinetically inert metal–ligand corner, followed by forming the rest of the structure using the pre-assembled building block or metal cation exchange. While this approach tends to be effective, it involves two steps, requiring more synthetic effort than homometallic metallo-supramolecular one-pot self-assembly. A small number of one-pot self-assembly examples of heterobimetallic cages have been reported,[25, 35, 44-47] employing the two distinct coordination geometries of two different metal cations.
Here, we present a diazocine-based ligand (L) that self-assembles into various metallo-supramolecular MII2L helicates in the presence of different metal(II) cations (ZnII, FeII, and CoII). The ligand features two chemically distinct coordination sites, allowing the formation of both homo- and heterobimetallic helicates (FeII2L, CoII2L, ZnII2L, ZnIIFeIIL, FeIIZnIIL, and ZnIICoIIL) with precise metal distribution achieved through one-pot self-sorting, sequential metal addition or metal exchange. Crucially, the reversible photoisomerisation of diazocines in L induces structural changes in the MII2L helicate, thereby shifting the assemblies away from the thermodynamic minimum, akin to a molecular ratchet. Continuous exposure to white light autonomously drives this molecular ratchet, leading to the formation of a single high-energy isomer with notable selectivity. Furthermore, we used this light-driven molecular ratchet to facilitate the dynamic and selective exchange of metal cations, transforming the homometallic Zn2L helicate into a heterobimetallic ZnFeL helicate.
Results and Discussion
Synthesis and Photoswitching of Ligand L
Photoresponsive subcomponent 1 was synthesised from 2-bromo-8-iodo-11,12-dihydrodibenzo[c,g][1,2]diazocine[48] (Scheme S1 and Section S2) via two successive, selective, and high-yielding cross-coupling reactions with ethyl pinacol boronic acid esters[49] 5-(4,4,5,5-tetraethyl-1,3,2-dioxaborolan-2-yl)-2,2′-bipyridine (S3) and 2-(1,3-dioxolan-2-yl)-5-(4,4,5,5-tetraethyl-1,3,2-dioxaborolan-2-yl)pyridine (S6).
At ambient conditions, subcomponent 1 primarily exists as its thermodynamically stable Z-isomer (99% Z-1). When exposed to 390 nm light, a second set of signals associated with E-1 emerged in the 1H NMR spectrum, reaching a photostationary state of 72% E-1 and resulting in a colour change of the solution from yellow to red (Scheme 1, left; Section S7.4). E→Z isomerisation (>99% Z-1) took place under white light or 500 nm light irradiation and through thermal relaxation, exhibiting a half-life of 165 min at 25 °C in acetonitrile (20 µM; Section S7.3.1). The photoswitching of subcomponent 1 is rapid and completely reversible, usually taking less than a minute of irradiation in either direction (20 µM–2 mM in CH3CN, 0.1–0.2 mW light power). The asymmetric 2,8-substitution pattern at the central diazocine unit in subcomponent 1 enables a significant structural change from a 90° to a 180° angle between the two metal-binding moieties upon photoswitching (Scheme 1, left; Figure S67). Employing two distinct metal-binding moieties generates directionality within asymmetric subcomponent 1. Dynamic covalent tethering of 1’s pyridine carboxaldehydes into TREN tri-pyridylimines fixes subcomponent 1’s orientation, leading to regioselective helicate self-assembly, as previously demonstrated[26] in related systems.
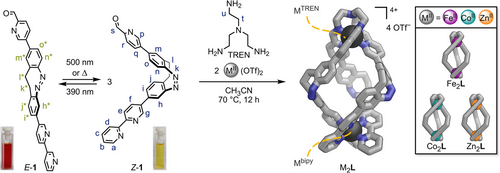
Synthesis of Homometallic Helicates
Homometallic, triple-stranded helicates Fe2L, Co2L, and Zn2L were obtained from the self-assembly of pyridine carboxaldehyde 1 (1 equiv.), tris(2-aminoethyl)amine (TREN, 1 equiv.), and the corresponding metal(II) trifluoromethanesulfonates (triflate or −OTf, 2 equiv.), Fe(OTf)2, Co(OTf)2, and Zn(OTf)2, respectively (Scheme 1, right). Fe2L, Co2L, and Zn2L were characterised by mass spectrometry, NMR spectroscopy, and UV–vis spectroscopy (Sections S3 and S7.3).
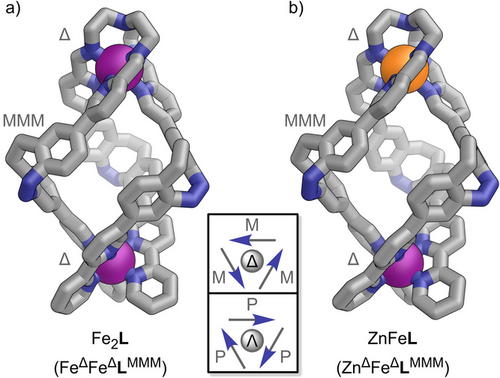
Fe2L crystallised in the triclinic space group P1 (Figure 1a; Section S6.2). Triple-stranded ligand L connects two homochiral metal centres, forming a dinuclear helicate structure with distorted C3 symmetry. All Z-diazocine units maintain the same orientation within ligand L, with their helical configuration (LMMM) opposing the helicity of the metal centres (FeΔΔ, Figure 1a; Sections S6.1 and S6.2). The self-assembly of dinucelar homometallic helicate Fe2L exhibits remarkable diastereoselectivity, resulting in the formation of only one pair of enantiomers: FeΔFeΔLMMM and FeΛFeΛLPPP.
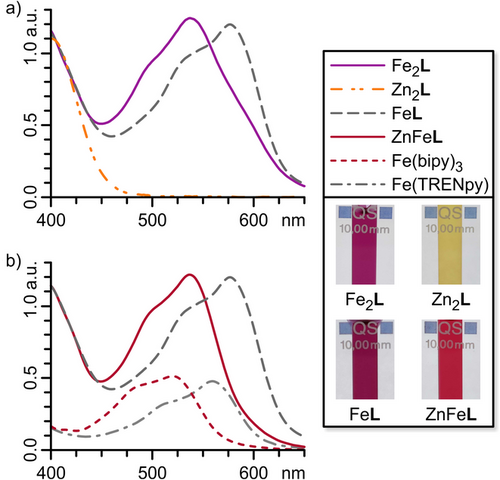
The high-resolution electrospray ionisation (ESI) mass spectrum of Fe2L showed a sequence of signals corresponding to the species [Fe2L − nOTf]n+ (n = 1, 2, 3, and 4), confirming the stoichiometry of Fe2L in the gas phase (Figures 2a and S24). 1H NMR spectroscopy indicated a C3 symmetric structure with diasterotopic signals for the TREN ethylene groups, as expected for the chiral coordination pocket (Figures 2b and S19). Proton signals of H-p, H-s, H-u′, and H-t′ close to the TREN tri-pyridylimine metal corner (FeTREN) are significantly broadened with half-widths of 30 to 50 Hz and are only visible in highly concentrated samples (Figure S19). 1H,1H ROESY spectroscopy revealed close interproton contacts between diazocine protons H-h and H-m with bipyridine proton H-g and pyridylimine proton H-p, respectively, confirming that the same racemic diastereomer of Fe2L, namely FeΔFeΔLMMM, together with its enantiomer FeΛFeΛLPPP, is present in both solution and the solid state (Figure S56 and Section S6.4.1). The UV–vis spectrum of Fe2L displays a diazocine n–π* absorption band at 405 nm, along with three bands in the visible range that are characteristic of FeII MLCT transitions (λ = 497, 539, and 599 nm; Figure 3a). By synthesising model compounds for the two unique metal binding sites in Fe2L—Fe(bipy)3(OTf)2 and Fe(TREN tri-pyridylimine)(OTf)2 (short: Fe(bipy)3 and Fe(TRENpy), respectively)—and comparing their UV–vis spectra to that of Fe2L (Figure 3b), we assigned the three MLCT transition bands to the two distinct coordination sites in Fe2L. The two lower-wavelength bands are attributed to the MLCT transition of one FeII coordinated by three bipyridines (Febipy, λbipy = 497 and 539 nm). The second FeII coordinated by the TREN tri-pyridylimine coordination pocket (FeTREN) causes the much weaker higher-wavelength band at λTREN = 599 nm. These findings align well with comparable FeII complexes documented in the literature.[50, 51]
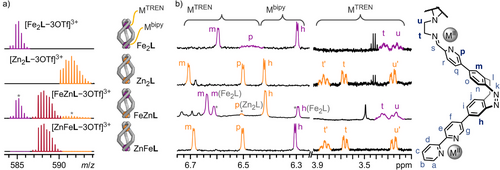
Characterisation of homometallic helicates Co2L and Zn2L by mass spectrometry and NMR spectroscopy indicated the formation of similar structures to Fe2L (Figures 2 and 3; Sections S3.3, S3.4 and S6.4.2).
The 1H NMR spectrum of Co2L is characteristically dispersed over a range of nearly 200 ppm (Figure S26), due to the paramagnetism of CoII, which precludes further assignments of the signals. The UV–vis spectrum of Co2L shows an MLCT absorption band at λ ≈ 350 nm and an almost unchanged diazocine n–π* absorption band (405 nm; Figure S72).
The 1H NMR spectrum of Zn2L showed signal shifts similar to but clearly distinct from Fe2L (Figure 2b). UV–vis spectroscopy of Zn2L revealed an almost unchanged diazocine n–π* absorption band around 405 nm compared to subcomponent 1, with no MLCT transition observed in the visible range (Figures 3a and S70).
The characteristic proton shifts of H-h, H-m, H-p, H-t, and H-u of Fe2L and Zn2L in 1H NMR (Figure 2b), as well as the three characteristic MLCT bands of Febipy and FeTREN in UV–vis (Figure 3a), allow for detailed elucidation of FeII and ZnII distribution between the two coordination sites of ligand L, enabling us to analyse heterobimetallic helicates precisely.
Synthesis of Heterobimetallic Helicates
Ligand L features two coordination sites that are chemically, kinetically, and thermodynamically distinct: a TREN tri-pyridylimine and a bipyridine. The sixfold chelation of the TREN tri-pyridylimine coordination pocket (MTREN) is kinetically more inert and potentially thermodynamically preferred over the three twofold chelating bipyridines (Mbipy) due to an enhanced chelate effect similar to that of a cryptand. The greater stability of TREN tri-pyridylimine coordination sites, compared to three individual pyridylimines, has been used in previous applications for subcomponent exchange and cage stabilisation.[52, 53] Considering kinetics, the bipyridine binding site (Mbipy) should be accessible first, as the TREN tris-pyridylimine coordination pocket (MTREN) has not yet formed via metal-mediated imine-bond formation. Thus, employing ligand L alongside two different metal cations, with one forming thermodynamically more stable M─N bonds[54] and exhibiting slower ligand exchange kinetics,[55-57] should result in the selective assembly of heterobimetallic helicates. Potential combinations of metal cations that favour an octahedral coordination sphere include FeII and ZnII or CoII and ZnII.
Using the greater stability and kinetic inertness of the TREN tri-pyridylimine binding pocket (MTREN), we attempted the consecutive assembly of a heterobimetallic helicate by mixing pyridine carboxaldehyde 1 (3 equiv.), TREN (1 equiv.), and Fe(OTf)2 (1 equiv.) to form mononuclear complex FeL with FeII in the TREN tris-pyridylimine binding pocket (FeTRENL, Figure 4a). Subsequently, Zn(OTf)2 (1 equiv.) was added to FeL to allow formation of a heterobimetallic helicate with FeII remaining in the TREN tris-pyridylimine binding pocket (FeTREN) and ZnII coordinating to the three bipyridine units (Znbipy), which we refer to as FeZnL (short for FeTRENZnbipyL). High-resolution ESI mass spectrometry confirmed the formation of an [Fe + L] species following the first reaction step, with minor amounts of Fe2L also detected. After the second reaction step, an [Fe + Zn + L] species was generated, with some Fe2L still present alongside newly formed Zn2L (Figures 2a and S31). The presence of both Fe2L and Zn2L, alongside the desired FeZnL, suggests that the formation of Fe2L is thermodynamically more favourable than the formation of FeL, which results in unreacted or partially reacted TREN and 1 remaining in the reaction mixture. After the addition of Zn(OTf)2, the unreacted or partially reacted ligand subcomponents, TREN and 1, form Zn2L.
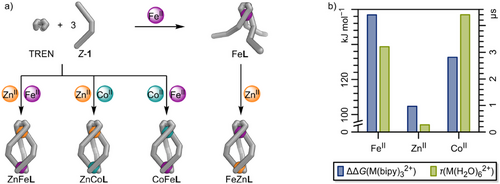
The 1H NMR spectrum of FeZnL confirmed the formation of a heterobimetallic helicate after the second reaction step (Figures 2b and S30). The chemical shifts of protons near the TREN tris-pyridylimine binding pocket, specifically diazocine proton H-m, pyridylimine proton H-p, and ethylene protons H-t and H-u, are comparable to the chemical shifts of their Fe2L counterparts. In contrast, the chemical shift of diazocine proton H-h, which is close to the bipy unit, is nearly identical to that of its Zn2L counterpart, indicating an FeTRENZnbipyL structure. As indicated by the ESI mass spectrum, 1H NMR spectroscopy also suggested the formation of dinuclear homometallic Fe2L and Zn2L (25% and 10%, respectively, Figure 2b; Section S3.7). The higher proportion of Fe2L compared to Zn2L is likely due to an excess of Fe(OTf)2 (approximately 10%) present during the formation of FeL. As described above, distinct MLCT absorption bands for FeII, coordinated by three bipyridine units (Febipy) or a TREN tris-pyridylimine binding pocket (FeTREN), facilitated further structural conformation of FeL and FeZnL using UV-vis spectroscopy (Figures 3 and S32). The UV–vis spectra of FeTRENL and the reference compound Fe(TRENpy) are nearly identical, suggesting the presence of an FeTREN coordination sphere in FeTRENL. The UV–vis spectrum of FeZnL reveals a secondary band associated with the additional MLCT transition observed in the contaminant Fe2L. Still, it displays a major band that is identical to the one found in FeTRENL. The difference in absorption bands between Febipy and FeTREN is readily visible to the naked eye, as the bipyridine-containing FeII complexes exhibit a vibrant red colour, whereas their pyridylimine counterparts present a deep purple hue (Figure 3, insert). The kinetics of FeTRENL complex formation were further investigated using UV–vis spectroscopy (Section S5), which indicated the nearly immediate formation of an Febipy13 complex, in which FeII is coordinated by three bipy units, as evidenced by the appearance of the characteristic Febipy-MLCT absorption band. Over a few hours, the Febipy-MLCT absorption band transformed into an FeTREN-MLCT absorption band, thus indicating the successful formation of FeTRENL. The progress of the reaction can also be observed with the naked eye: the reaction solution turns a distinctive Febipy-red colour within minutes and then gradually changes to a deep violet shade, characteristic of FeTREN, indicating the sequential formation of Febipy13 and FeTRENL. Therefore, we conclude that Febipy13 is kinetically favoured, whereas FeTRENL is potentially thermodynamically more stable but also kinetically more inert, thus confirming our assumption that the TREN tris-pyridylimine coordination pocket should be the more stable binding site.
After confirming our assumption regarding the kinetic and thermodynamic coordination behaviours of aldehyde 1 and ligand L, we aimed to harness these properties to achieve heterobimetallic social self-sorting using FeII, ZnII, and CoII. Given that FeII and ZnII show the most significant difference in thermodynamic and kinetic stabilities (Figure 4b), we initially sought to form a self-sorted ZnFeL heterobimetallic helicate (short for ZnTRENFebipyL) from a mixture of pyridine carboxaldehyde 1 (3 equiv.), TREN (1 equiv.), Fe(OTf)2 (1 equiv.), and Zn(OTf)2 (1 equiv.) in acetonitrile (Figure 4a). The self-assembly of FeTRENL indicated that the Mbipy coordination site would form before the MTREN binding site because it is readily accessible. In contrast, the MTREN coordination site must be formed through three metal-mediated imine condensation reactions, which occur on a significantly slower timescale. Consequently, we anticipated that both FeII and ZnII would first coordinate to the bipy units of 1, with FeII gradually replacing the thermodynamically less stable ZnII (ΔΔGFe,Zn(M(bipy)32+) = 52.2 kJ mol−1)[54] at all coordination sites, resulting in the formation of Febipy13. Once all FeII is coordinated to bipy (Febipy), only ZnII should remain to facilitate the formation of TREN tri-pyridylimine, thereby selectively installing ZnII in the MTREN coordination pocket to produce ZnFeL (short for ZnTRENFebipyL). ZnFeL crystallised in the triclinic space group P1 (Figure 1b; Section S6.2). The solid-state structure confirmed the expected ZnTREN-Febipy metal distribution in a triple-stranded helicate structure almost identical to that of Fe2L (Figure 1a; Section S6.2). High-resolution ESI mass spectrometry confirmed the formation of a [Zn + Fe + L] heterobimetallic helicate (Figures 2a and S38). 1H NMR spectroscopy indicated the expected regioselectivity of the self-sorted helicate ZnFeL (ZnTRENFebipyL), as evidenced by the characteristic proton signal shifts of protons H-h, H-m, H-p, H-t, and H-u corresponding to ZnTREN and Febipy coordination (Figure 2b). Furthermore, 1H,1H ROESY NMR spectroscopy confirmed the retention of the solid-state structure in solution (Section S6.4.1). UV–vis spectroscopy revealed a characteristic Febipy-MLCT absorption band (λbipy = 537 nm), whereas no FeTREN-MLCT absorption band (λTREN ≈ 580 nm) was detected, further supporting the successful self-sorting within ZnFeL (Figures 3b and S76).
The investigation of the kinetics of ZnFeL complex formation via UV–vis spectroscopy further corroborated our proposed assembly mechanism for ZnFeL (Section S5). An Febipy-MLCT absorption band (λbipy = 540 nm) quickly appeared, with no further changes to the spectra, indicating that FeII remained in the bipy binding site, thereby enabling the selective formation of TREN tri-pyridylimine around ZnII. These findings suggest that this self-sorting into ZnFeL is, indeed, a delicate interplay between ligand association and formation kinetics as well as metal-coordination thermodynamics. Prolonged heating of ZnFeL (65 °C, 2 months) in the presence of 3.0 equiv. FeII led to the formation of 6% Fe2L, demonstrating the kinetic inertness of the ZnFeL structure and the ZnTREN coordination site while indicating that Fe2L might be thermodynamically favoured over ZnFeL (Section S9.4).
To support our hypothesis that heterobimetallic helicate self-sorting occurs due to a delicate interplay between metal–ligand stability and exchange kinetics, we attempted the synthesis of two additional heterobimetallic helicates using ZnII/CoII and CoII/FeII mixtures. In these mixtures, both the differences in thermodynamic metal–ligand stability and in metal–ligand exchange kinetics are gradually reduced. While the difference in metal–ligand exchange kinetics between ZnII and CoII is comparable to that of the ZnII/FeII pair,[55-57] the difference in Mbipy-association energy between Co(bipy)32+ and Zn(bipy)32+ is approximately half as high as that between Fe(bipy)32+ and Zn(bipy)32+ (ΔΔGFe,Zn(M(bipy)32+) = 52.2 kJ mol−1 and ΔΔGCo,Zn(M(bipy)32+) = 28.0 kJ mol−1, respectively; Figure 4b).[54] Therefore, helicate self-assembly from a mixture of ZnII and CoII should indicate whether the difference in thermodynamics or kinetics of the metal cations has a more significant impact on successful self-sorting. For CoII and FeII, both the difference in Mbipy-association energy between Co(bipy)32+ and Fe(bipy)32+ and the difference in metal–ligand exchange kinetics are small.[54-57] Consequently, if our hypothesis was correct, we expected poor self-sorting, if any.
In the case of the one-pot heterobimetallic helicate assembly involving ZnII, CoII, 1, and TREN (Figure 4a), both 1H NMR spectroscopy and ESI mass spectrometry indicated selective self-sorting into the ZnCoL (ZnTRENCobipyL) helicate (SI,Section S4.1.2). ESI mass spectrometry revealed a singular set of peaks for [Zn + Co + L + nOTf](4−n)+ (n = 0, 1, 2), and the wide-sweep 1H NMR spectrum displayed only signals reminiscent of those in Co(bipy)3(OTf)2, with none reminiscent of a CoII-TREN tri-pyridylimine coordination.
Helicate assembly in the presence of CoII and FeII, however, resulted in a mixture of CoFeL (CoTRENFebipyL, approx. 60%–70%), Fe2L (approx. 15%) and Co2L helicates (approx. 15%). The composition of the helicate mixture was tentatively estimated using ESI mass spectrometry, NMR, and UV–vis spectroscopy (Sections S4.3 and S4.4). The paramagnetic nature of CoII hindered the determination of accurate values.
-
Both metal cations form Mbipy-complexes with three equivalents of subcomponent 1, respectively.
-
Because of the disparity in metal–ligand binding energies (FeII > CoII > ZnII), the more stable metal cation replaces the weaker in the Mbipy complexes and gets sequestered by the Mbipy coordination sphere.
-
“Trapping” the more stable cation in the Mbipy coordination sphere allows the thermodynamically less stable metal cation to facilitate the formation of the TREN tri-pyridylimine binding pocket MTREN, thereby completing the self-sorted heterobimetallic helicate.
Photoswitching of Zn2L
After demonstrating the ability of ligand L to form either dinuclear homometallic helicates or even heterobimetallic ones via the effective self-sorting of two different metal cations, we investigated the effects of switching the photochromic diazocine units within the helicates.
Irradiation of an acetonitrile solution of Zn2L with visible light (405 nm, 25 °C, 1 mM) resulted in a colour change from yellow to red, along with the disappearance of the 405 nm absorption band and the emergence of an intense absorption band at 490 nm in the UV–vis spectrum (Figure 5a). The changes in the UV–vis spectrum are nearly identical to those observed for aldehyde 1 (Figure S80), suggesting an equally efficient Z→E conversion in Zn2L helicate as in free aldehyde 1 upon irradiation with 405 nm light. High-resolution ESI mass spectrometry demonstrates that the Zn2L composition remains intact following irradiation with 405 nm light (Figure S99). Additionally, 1H DOSY NMR spectroscopy indicated that the diffusion coefficients—and, therefore, the solvodynamic diameters—of the structures before and after 405 nm light irradiation differ only slightly (Figure 6b, bottom). In the 1H NMR spectrum, irradiation of Zn2L with 405 nm light results in the disappearance of nearly all previously sharp and well-defined signals, leaving only ill-defined and broad signals, apart from the signals of the ethylene groups H-t and H-u. Moreover, no signals for the free ligand L or free aldehyde 1 were detected, indicating that at least the ZnTREN binding pocket remained intact following irradiation (Figure 6b, green, 2nd from top). The intact ZnTREN binding pocket, along with high-resolution ESI mass spectrometry confirming the retention of the Zn2L composition, 1H DOSY NMR spectroscopy suggesting a structure of a similar solvodynamic diameter, and UV–vis spectroscopy demonstrating successful switching of the diazocine units, might indicate the formation of an E,E,E-Zn2L structure (abbreviated as E-Zn2L, Figure 6a). Although switching should occur in a stepwise manner, we tentatively assume structures in which only some of the diazocines are switched, namely E,E,Z-Zn2L and E,Z,Z-Zn2L, to be unlikely, as literature precedence[14, 18, 58, 59] of cages containing photochromic units in their ligand backbone suggests that switching is a highly cooperative process, with intermediates only observed at extremely low temperatures.[60] The first switching event causes the most significant structural change, potentially accelerating the subsequent switching of other photochromic units within the same structure to relieve strains caused by previous isomerisation events.
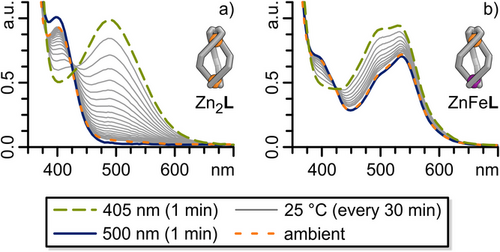
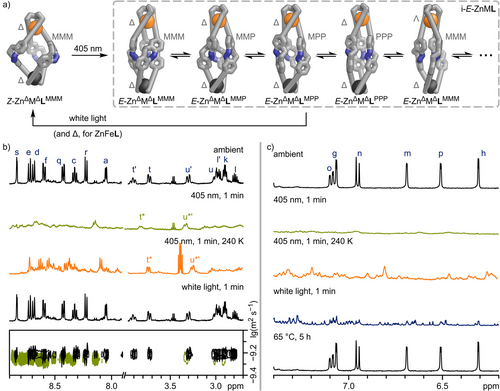
The observed broad 1H NMR signals after 405 nm irradiation of Zn2L (Figure 6b, green, 2nd from top) indicate structural transformations, which are on intermediate exchange on the NMR timescale. We tentatively assume these to be hindered rotations of the diazocine moieties within the arms of the ligands or isomerisation of the helicate to a pseudo-mesocate. In E,E,E-Zn2L, the three “arms” of ligand L adopt a pseudo-linear configuration, enabling rotation of the diazocine moieties (i-E-Zn2L, Figure 6a, right; Figure S102).
Rotation of the diazocine moieties would give rise to four potential constantly interconverting rotamers with the diazocine units pointing with their azo-nitrogens either in a clockwise (LPPP) or counterclockwise direction (LMMM) in the helicate or even mixtures of both (LPPM or LMMP; Figure 6a). Additionally, due to steric strains within E-Zn2L, we anticipate that both ZnII corners in E-Zn2L will be significantly weaker than those in Z-Zn2L, potentially promoting isomerisation between helicate and pseudo-mesocate or even facilitating metal exchange.
To test our hypothesis regarding the interconversion of rotamers and helicate-to-pseudo-mesocate leading to the broad signals in the 1H NMR spectrum after 405 nm irradiation of Z-Zn2L, we performed low-temperature 1H NMR spectroscopy (240 K) on the same sample (Figure 6b, orange, 3rd from top; Figure S97). The resulting spectrum reveals a multitude of sharp signals, indicating a plethora of chemical environments and isomeric structures, which do not interconvert at this temperature. This observation supports our hypothesis that multiple interconverting diastereomers (i-E-Zn2L) are responsible for the broad signals observed at 298 K. Irradiation of i-E-Zn2L with either 500 nm or white light reverses the process almost instantly, indicating that Zn2L switching is completely reversible at ambient temperature (Figure 6b, black, 4th from top). However, at 240 K, irradiation of i-E-Zn2L with white light does not lead to the clean formation of Z-Zn2L but rather a multitude of sharp signals that indicate the formation of multiple isomers (Figure S97). Therefore, we tentatively assume that irradiation with white light causes all diazocine moieties to switch back into their Z states, with each i-E-Zn2L helicate or pseudo-mesocate rotamer isomerising to its corresponding i-Z-Zn2L helicate or pseudo-mesocate atropisomer. These atropisomers cannot interconvert without breaking at least one pair of ZnII-bipy coordinative bonds. At 240 K, the abundance of atropisomers results in numerous sharp 1H NMR signals (Figure S97). At room temperature, the ZnII-bipy coordinative bond readily dissociates, allowing the atropisomers to converge to the most stable Z-Zn2L structure (Figure S97). Thus, the isomerisation process Z-Zn2L→E-Zn2L→Z-Zn2L is influenced by the kinetic lability of the metal cation within the bipy pocket of L. To further explore the intricate Z-Zn2L→E-Zn2L→Z-Zn2L switching mechanism and support our hypothesis, we investigated switching in ZnFeL, which contains a kinetically more stable FeII in the bipy binding site of L (Febipy) and should, thus, hinder isomerisation of the atropisomers (i-Z-ZnFeL) to the thermodynamically preferred structure (Z-ZnΔFeΔLMMM).
Photoswitching of ZnFeL: A Molecular Energy Ratchet
Similar to Zn2L, the irradiation of ZnFeL with 405 nm light resulted in a 1H NMR spectrum featuring broad signals, while 1H DOSY NMR spectroscopy and high-resolution ESI mass spectrometry indicated the formation of a species with the same composition and a similar size to Z-ZnFeL, which we denote as i-E-ZnFeL (Figure 6; Sections S7.4 and S7.5). The UV–vis spectra of Z-ZnFeL and E-ZnFeL showed nearly the same differences as those between Z-Zn2L and E-Zn2L, as well as Z-1 and E-1, with the MLCT absorption band remaining unchanged (Figure 5b; Figure S80). This suggests that photoswitching remains efficient, even in the presence of the Febipy MLCT absorption band, and that both FeTREN and Febipy coordination are preserved. As with i-E-Zn2L, the observed broad 1H NMR signals for i-E-ZnFeL sharpened when cooled to 240 K (Figure 6c, orange, 3rd from top), indicating that diazocine rotation and helicate→pseudo-mesocate isomerisation caused the broadened signals at room temperature.
As envisioned, irradiating i-E-ZnFeL with white light resulted in a complex 1H NMR spectrum featuring multiple signal sets (Figure 6c, blue, 4th from top). This signal pattern is similar to that of i-E-Zn2L following 405 nm and white light irradiation at 240 K, prompting us to label it i-Z-ZnFeL (Scheme 2; Figure S98). We can reform the thermodynamically stable Z-ZnFeL by heating i-Z-ZnFeL at 65 °C for five hours (Figure 6c, black, 5th from top). The formation of kinetically trapped states after 405 nm and white light irradiation at room temperature was also observed for Fe2L (Figure S84), supporting our hypothesis that the dissociation of bipyridine units in the Mbipy coordination site is the rate-determining step for the kinetically trapped states to revert to the thermodynamically favoured Z-MΔMΔLMMM (Z-ZnΔZnΔLMMM, Z-ZnΔFeΔLMMM, and Z-FeΔFeΔLMMM, respectively). The stronger metal–ligand bond of FeII compared to ZnII leads to a higher barrier for the dissociation of metal–bipyridine coordination, creating a more effective kinetic trap. This elevated barrier is evident in the temperature needed for the various helicate and pseudo-mesocate atropisomers (i-Z-Zn2L, i-Z-ZnFeL, and i-Z-Fe2L) to return to their initial thermodynamically favoured states Z-MΔMΔLMMM (Z-ZnΔZnΔLMMM, Z-ZnΔFeΔLMMM, and Z-FeΔFeΔLMMM, respectively), which rises from room temperature for i-Z-Zn2L to 65 °C for i-Z-ZnFeL and i-Z-Fe2L.
We examined the kinetically trapped intermediates during the switching cycle of ZnFeL through in situ illuminated 1H NMR spectroscopy (Section S7.4.3). Although the variety of signals hindered a thorough kinetic analysis, our findings indicate that irradiating ZnFeL with 405 nm light produces a mixture of at least two distinct species. This implies that the structural alterations induced by 405 nm light irradiation result in a distribution of conformational and possibly isomeric (helicate and pseudo-mesocate) states. The notable asymmetry in these i-E-ZnFeL states is evident in the intricate 1H NMR spectrum.
-
Irradiation of ZnFeL with 405 nm light causes all diazocine units within the structure to isomerise to their E-configurations, forming symmetric E-ZnFeL (E-ZnΔFeΔLMMM).
-
In E-ZnFeL, the diazocines can now rotate, albeit slowly, resulting in four rotamers (E-ZnΔFeΔLMMM, E-ZnΔFeΔLPMM, E-ZnΔFeΔLPPM, E-ZnΔFeΔLPPP; Figure 6a; Section S7.6). E-ZnFeL is sterically strained with weakened coordinative bonds around the ZnTREN and Febipy corners, which allows for helicate→pseudo-mesocate isomerisation, giving rise to four pseudo-mesocate rotamers (E-ZnΛFeΔLMMM etc.; Sections S6.3 and S8). Rotamer and pseudo-mesocate formation from symmetric E-ZnΔFeΔLMMM to i-E-ZnFeL occurs rapidly, indicating low energy differences and activation barriers between the states.
-
Irradiating the isomeric mixture i-E-ZnFeL with 500 nm or white light causes all diazocine units within the supramolecular structures to isomerise to their Z-configurations, trapping the respective helicates, pseudo-mesocates, and their rotamers (i-Z-ZnFeL).
-
Due to the kinetic inertness of FeII, i-Z-ZnFeL represent kinetically trapped states that can revert to the thermodynamically favoured Z-ZnΔFeΔLMMM by partial Febipy coordination site dissociation at 65 °C, thus closing the reaction cycle.
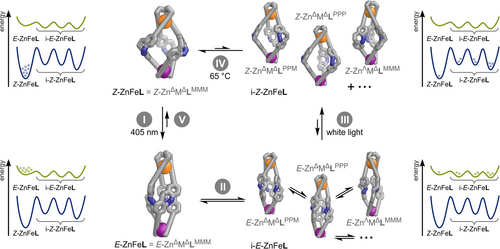
Trapping the respective i-E-ZnFeL in their current isomeric forms through photoswitching provides a potential direct pathway from symmetric E-ZnΔFeΔLMMM to ground state Z-ZnΔFeΔLMMM (Scheme 2, process V).
In the case of Zn2L, i-Z-Zn2L are kinetically trapped at low temperatures (Figure 6b), demonstrating that the same processes operate during the irradiation of Zn2L.
Since most molecules follow the reaction cycle unidirectionally (Scheme 2, processes I–IV), the photoswitching of ZnFeL acts as a light-driven molecular energy ratchet: 405 nm light irradiation drives the thermodynamically stable Z-ZnFeL helicate into metastable E-ZnFeL helicates, which can readily access various conformers and stereoisomers (i-E-ZnFeL). Under white light irradiation, these i-E-ZnFeL states relax swiftly into their corresponding kinetically trapped i-Z-ZnFeL isomers. These higher-energy kinetically trapped isomers (i-Z-ZnFeL) cannot readily return to the initial thermodynamically stable state (Z-ZnFeL) due to the required rotation of the diazocine units being only possible through partial Febipy coordination-site dissociation. Thus, the system captures light energy to convert the more stable isomer (Z-ZnFeL) into less stable, kinetically trapped isomers (i-Z-ZnFeL). This transformation leads to the occupancy of higher-energy states, storing some of the absorbed light energy as strained or unfavourable molecular conformations.
An Autonomously Operating Molecular Ratchet Under White Light Irradiation
-
Photochemical Z→E isomerisation of a Z-ZnFeL to an E-ZnFeL isomer, which subsequently isomerises into the corresponding E-ZnΛFeΔLMMM pseudo-mesocate. E→Z isomerisation then yields Z-ZnΛFeΔLMMM.
-
Slow thermal relaxation of less favourable high-energy i-Z-ZnFeL isomers to relatively more favourable pseudo-mesocate Z-ZnΛFeΔLMMM.
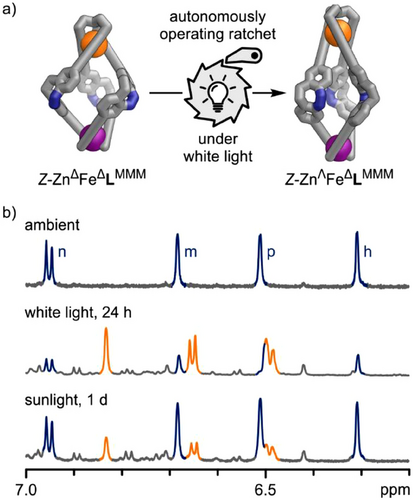
Kinetic studies on the thermal relaxation of the i-Z-ZnFeL mixture, obtained via subsequent 405 nm and white light irradiation, support the thermal relaxation pathway (ii). The time-dependent 1H NMR spectra revealed that various components of this mixture were interconverting, with none of the involved species returning to the initial ground state of Z-ZnFeL (Z-ZnΔFeΔLMMM; Section S7.4.4). Thermal conversion of pseudo-mesocate Z-ZnΛFeΔLMMM into the overall thermodynamically more stable Z-ZnΔFeΔLMMM helicate occurred at room temperature, exhibiting a half-life of several days (Figure S115).
Curiously, the molecular ratchet functions in sunlight as well. When a sample of Z-ZnΔFeΔLMMM helicate is placed by a window for one winter day, it transforms into a mixture enriched with pseudo-mesocate Z-ZnΛFeΔLMMM (Figure 7b, bottom). However, this process is less efficient compared to using a white LED. Therefore, the molecular ratchet is capable of converting light into chemical energy and can even retain that energy for a limited duration.
Metal Exchange
As mentioned earlier, ligand L contains two coordination sites with distinct kinetic stabilities, which we have shown to be essential for self-sorting of heterobimetallic helicates. To take advantage of the differences in kinetic inertness between the two binding sites, we performed a selective metal-cation exchange in the Mbipy-coordination site, transforming Zn2L into ZnFeL via ZnII→FeII exchange (Figure 8, top). Specifically, treating an acetonitrile solution of Zn2L with Fe(OTf)2 (1.5 equiv.) at 65 °C led to the formation of ZnFeL, confirmed by 1H NMR, UV-vis spectroscopy, and ESI mass spectrometry (Section S9). Kinetic studies using 1H NMR spectroscopy (Section S9.3) indicated that the ZnII→FeII exchange does not follow apparent first-order kinetics. Rather, Zn2L is rapidly consumed, resulting in both the intended product ZnFeL and a non-productive intermediate. This resting state is subsequently converted into the product ZnFeL via Zn2L as an intermediate.
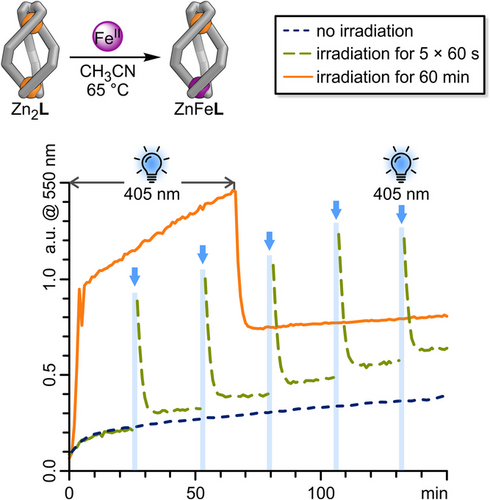
Given that the helicate molecular ratchet is accumulating high-energy structures (i-E-ZnFeL), in which the Mbipy coordination site is likely weakened, we aimed to examine whether we could use this unidirectional reaction cycle (Scheme 2) to expedite the ZnII→FeII cation exchange within Zn2L, resulting in the formation of ZnFeL. To evaluate this hypothesis, Fe(OTf)2 (1.5 equiv.) was added to an acetonitrile solution of Zn2L at 65 °C, and the mixture was illuminated with 405 nm light for 60 seconds every 25 min. Since ZnFeL shows a distinct Febipy MLCT transition band at 550 nm, which correlates with the number of Fe─N bonds formed and which Zn2L entirely lacks (Figure 5), this reaction can also be monitored in situ using UV–vis spectroscopy (Figure 8). Note that E-diazocines exhibit strong absorption at 550 nm (Figure S68), and this will influence the absorption band at 550 nm during irradiation, continuing until the diazocine thermally returns to its Z-form. The thermal relaxation of the E-diazocine moieties to their Z-configurations occurs rapidly at 65 °C (τ½(65 °C) = 72 s), achieving complete conversion within less than 10 min. In the dark, the Zn2L→ZnFeL background reaction (Figure 8, blue dotted line) shows a rapid initial rise in conversion before stabilising to a nearly linear reaction rate after about 12 min. Irradiating the Zn2L→ZnFeL reaction mixture with 405 nm light outside the UV–vis spectrometer results in a significant increase in absorption at 550 nm (Figure 8, green dashed line), primarily attributed to the absorption of the E-diazocine units. However, the observed increase in 550 nm absorption approximately 10 min after irradiation can be solely attributed to an increased Febipy absorption, which reflects the Zn2L→ZnFeL conversion. Four subsequent irradiations of the reaction mixture with 405 nm light further accelerate the conversion of Zn2L to ZnFeL, resulting in nearly double the amount of ZnFeL produced compared to the background reaction after 150 min. The increased conversion of Znbipy to Febipy after five 60-s light irradiations of the ZnFeL-Fe(OTf)2 reaction mixture reinforces our hypothesis that the molecular ratchet can help promote this exchange.
Notably, the Znbipy→Febipy exchange could also be accelerated under constant irradiation (60 min; Figure 8, orange solid line). Once the E-diazocine moieties have reached their photostationary states, their absorption remains constant under continuous irradiation. Consequently, the significant fivefold steeper increase in absorption observed between minutes 10 and 60, compared to the background reaction, can be solely ascribed to an accelerated exchange from Znbipy to Febipy.
We believe that the molecular energy ratchet mechanism is vital for enabling the metal-cation exchange process. By populating higher-energy states and enriching non-ideal conformers, the photoswitching process effectively destabilises the coordination sphere around the metal centres, thereby accelerating the Znbipy→Febipy exchange process. The ability to control this process through light irradiation offers a powerful tool for modulating kinetics in complex systems.
Conclusion
To summarise, we have created a unique photoswitchable diazocine-based ligand L, which self-assembles into a range of photo-responsive metallo-supramolecular bimetallic helicates. The subtle balance between bond strength and kinetic lability at both the ligand coordination sites and the metal cations enabled us to form dinuclear heterobimetallic ZnII-FeII and ZnII-CoII helicates with precise metal distribution through one-pot self-sorting reactions. We believe that this self-sorting primarily involves a kinetic selection process regarding ligand L’s binding sites, meaning that the Mbipy coordination site is formed first. Additionally, it entails a thermodynamic selection for the metal cations, where the more thermodynamically stable Fe13 (or Co13) complex is favoured over the less stable Zn13.
Importantly, we discovered that Z→E photoisomerisation of the diazo units in the metallo-supramolecular Zn2L and ZnFeL helicates initiates various conformational and stereochemical changes, all while preserving the overall constitutional integrity of the complexes. These conformers become trapped in higher energy isomers and atropisomers following E→Z isomerisation. We interpret the system as a molecular energy ratchet, capable of temporarily storing light energy by populating these energy-rich isomers. The molecular ratchet could autonomously function under continuous white light exposure, efficiently converting light energy into chemical energy within a high-energy Z-ZnFeL pseudo-mesocate.
The higher-energy E-isomers formed under light irradiation exhibit a more labile Mbipy coordination sphere, allowing the molecular ratchet to operate as a light-controlled accelerator for the highly selective metal-cation exchange process from homobimetallic Zn2L to heterobimetallic ZnFeL helicate.
Our work enhances the structural complexity and functionality of photoswitchable complexes, highlighting the potential of using light to drive supramolecular systems out of equilibrium. This could open up new paths for developing light-controlled molecular machines, stimuli-responsive catalysis, and photo-responsive building blocks for smart materials, potentially with customised energy storage and release characteristics.
Supporting Information
The authors have cited additional references within the Supporting Information.[61-82]
Author Contributions
M. J. N.: Synthesis; characterisation; photoswitching studies; analysis of kinetics; calculations of molecular models; and project conception. G. S.: X-ray crystallography. L. K. S. v. K.: Project conception and supervision.
Acknowledgements
This work was supported by the Fonds der Chemischen Industrie (FCI, Liebig Fellowship) and the German Research Foundation (DFG; Emmy Noether Programme, 446317932). M.J.N. thanks the FCI for a Ph.D. Fellowship and U. Weynand and Dr. S. Nozinovic for support with NMR measurements. M.J.N. and L.K.S.v.K. thank Dr B.M.W. Roberts for the helpful discussions regarding the fitting of kinetic data. G.S. thanks Prof. Dr. A.C. Filippou for providing X-ray infrastructure.
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article. Deposition numbers 2442413 (for Fe2L) and 2442414 (for ZnFeL) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service (http://www.ccdc.cam.ac.uk/structures).




