On-Demand Photodegradable and Thermo-Reversible, Soft, Transparent Dithioacetal Hydrogels
Abstract
Stimuli–reversible, chemically cross-linked polymers capable of altering their physicochemical and mechanical properties on demand, upon application of external stimuli (e.g., light, temperature), are highly desirable for the development of multifunctional materials. Herein, we report a facile chemical platform for the synthesis of photodegradable and thermo-reversible, model hydrogels consisting of poly(ethylene glycol) (PEG) as the elastic strands and dithioacetal moieties at the cross-link points. The gels were synthesized via an acid-catalyzed step-growth reaction of a difunctional PEG-thiol macromer with a wisely selected aromatic dialdehyde cross-linker. The formation of the photosensitive dithioacetal bonds at the cross−links rendered the hydrogels photodegradable, whereas the production of the initial comonomers as the main photoproducts after irradiation endowed the material with thermoreversible properties. The linear viscoelastic behavior of water-swollen gels, their photodegradation under UV (λ = 254 nm) irradiation at very low intensity (0.063 mW cm−1), and the reversible reformation of the hydrogel upon heating were investigated by dynamic shear rheology. Mechanistic insights for the photodegradation mechanism of the system were gained by 1H NMR spectroscopy and kinetic studies on a model dithioacetal compound.
Introduction
Covalently cross-linked thermoplastic polymers represent a class of materials that have been extensively employed in a variety of applications ranging from plastic packaging, coatings, adhesives, and biomaterials. However, conventional cross-linked polymers comprise strong covalent bonds, which reduce or even hinder their usefulness in some applications, for instance, in recycling and reprocessing the material at the end of the product lifetime, contributing to today's plastic waste crisis.
Covalent adaptable networks (CANs), consist of dynamic (reversible) covalent bonds within the polymer network, hence they can reversibly preserve their chemical and topological integrity through inherent dynamic bond exchange or stimuli-triggered (e.g., heat, pH, light) bond cleavage/bond formation reactions, facilitating the material malleability, reuse and multifunctionality[1, 2] Multifunctional polymeric CANs represent an appealing class of materials for use in emerging fields, such as soft robotics and electronic skin.[3-5] Depending on the mechanism of dynamic bond exchange, CANs can be “associative” or “dissociative.” In associative CANs (or vitrimers) there is a continuous bond-breaking/reformation sequence (reversible exchange reactions), and thus the two chemical processes cannot be observed separately. As a result, associative CANs preserve their network integrity without changes in their rheological behavior (viscosity), and the network cannot undergo a gel-to-sol transition in the temperature range in which the bonding energy is high, exhibiting features of both thermosets and thermoplastics. Examples of such associative covalent bonds include transesterification,[6] thiol-thioester,[7] urea-amine,[8] and siloxane.[9] On the other hand, the dissociative mechanism (reversible addition reactions) involves a rearrangement process, in which a bond is formed before the cleavage of another linkage.[2] Typical examples are the Diels-Alder,[10, 11] alkoxyamine[12, 13] and thiol-ene (thiol-Michael addition) reactions.[14, 15] In some cases, the mechanism of bond rearrangement can vary from associative to dissociative depending on the environmental conditions and/or the application of an external stimulus. For instance, disulfide, diselenide, (acyl)hydrazone, imine, and oxime bonds can undergo either exchange reactions or addition reactions depending on the experimental conditions.[2, 16-18]
Of interest are the stimuli-reversible polymer networks, which undergo a gel-to-sol transition upon bond cleavage using one type of stimulus (e.g., light, temperature), and the reverse process, which leads to network reformation by a second stimulus. In this respect, most studies rely on materials containing anthracene,[19, 20] coumarin[21] and cinnamoyl[22] moieties, for which the disintegration of the network and its subsequent reformation can be manipulated using temperature or/and light irradiation of different wavelengths as the external triggers. For example, Du Prez et al developed a thermo-degradable and photo-reversible network based on anthracene moieties.[20] Upon heating above 180 °C, a retro-cycloaddition reaction of the anthracene molecules was activated, leading to network degradation, whereas UVA irradiation induced the dimerization of anthracene and subsequent network reformation.
Due to the versatility and the unique advantages offered by light, including the precise spatiotemporal control of irradiation, and the diversity of the irradiation wavelength and intensity, photo-responsive or photodegradable covalent bonds are of particular interest.[23, 24] To this end, a plethora of functional photosensitive polymer networks with on-demand manipulation of their mechanochemical properties through light application have been developed for use in photopatterning,[25, 26] drug and cell delivery,[27, 28] and to study cell dynamics.[29-31] The synthesis of photocleavable hydrogels requires the incorporation of photo-sensitive bonds within the hydrogel structure.[32] Photo-sensitive moieties that have been employed in 3D polymer networks so far include the ortho-nitrobenzyl (o-NB) ester,[27, 31, 33-35] coumarin ester,[36] ONB triazole,[37] disulfide bonds,[16] and ruthenium complexes.[38] In 2009, Anseth's group presented for the first time the synthesis of a photodegradable poly(ethylene glycol) (PEG) diacrylate cross-linker and its subsequent copolymerization to obtain photodegradable PEG-based hydrogels.[33] Interestingly, the authors showed the successful photolysis of the hydrogel, upon single photon irradiation at 365 nm, with intensities between 20 and 10 mW cm−2, and 405 nm, with 25 mW cm−2, as well as by two-photon irradiation at 740 nm. Since then, the photodegradable PEG-diacrylate cross-linker has been extensively employed for the fabrication of chemically cross-linked hydrogels with temporally and spatially tunable physicochemical properties for use in various biotechnological applications.[26, 30, 39] However, in all these cases, the light intensity required for the hydrogel degradation was quite high, and the photoproducts obtained precluded the reformation of the polymer network.
The cleavage of dithioketal/dithioacetal bonds under oxidative conditions is a well-known chemical process, which has been extensively employed in organic synthesis. These dithioacetal linkages have been incorporated in linear polymers and hydrogels to yield materials with responsive properties towards reactive oxygen species for use in biomedicine.[40-43] The main synthetic strategies employed for the preparation of dithioketals/dithioacetals are the acetal exchange reaction with thiols,[41, 43] the thioacetalization of ketones and aldehydes,[44] and the base-catalyzed double thiol-Michael addition on alkynones.[45] Besides being an attractive synthetic route for dithioacetal preparation, the latter also confers a dynamic nature to the dithioacetal moieties via the thiol exchange reactions.
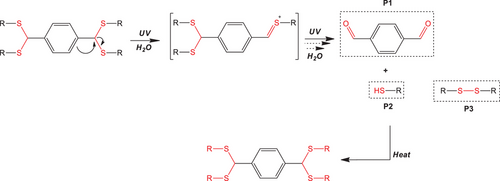
Our group, working extensively on the synthesis and characterization of linear photocleavable polymers, has introduced two new families of photodegradable polymers, namely, polyacetals[46, 47] and polyacylhydrazones.[48, 49] Herein, we demonstrate a new, facile chemical platform for the synthesis of multifunctional, stimuli-reversible hydrogels based on dithioacetal cross-links, with a very low degradation threshold. The dithioacetal cross-links endow the materials with on-demand photo-degradable and thermo-reversible properties upon application of two orthogonal stimuli, light irradiation and temperature. Inspired by the concept of sustained circular economy and the chemical recycling of polymers, we envisaged a polymer hydrogel capable of undergoing photocleavage to produce the initial (macro)comonomers, which can then be reactivated by the application of temperature to restore its chemical composition and viscoelastic properties. To this end, PEG-based networks cross-linked via dithioacetal linkages were synthesized via an acid-catalyzed step-wise polymerization mechanism. The thioacetalization reaction took place between the judiciously selected aromatic dialdehyde, terepthaldehyde (TPA), which served as the photo-absorbing unit, and dithiol terminated PEG chains, that bestowed the elastic strands of the network. The viscoelastic properties of water swollen hydrogels, the photodegradation process of the hydrogels under UV exposure at λ = 254 nm, and the reversible formation of the network upon heating were investigated by dynamic shear rheology. The photodegradation mechanism was investigated by means of kinetic studies using a model dithioacetal-based 4-arm star polymer. The rational selection of the comonomers, as well as the mechanism of network formation, allowed us to study and successfully demonstrate the photodegradable and thermo-reversible properties of this new system.
Results and Discussion
Synthesis of the PEG-Based Hydrogels
The synthesis of polymer networks via step-growth reactions require the use of a multi-functional comonomer, bearing at least 3 functionalities (f>2), to create the intermolecular cross-links. However, in the acid-catalyzed thioacetalization mechanism of aldehydes, a dialdehyde comonomer can serve as a tetra-functional cross-linker, forming dithioacetal linkages upon reaction with a dithiol comonomer. Taking into consideration this reaction mechanism, we aimed to synthesize PEG-based networks with dithioacetal moieties at the cross-links. The hydroxyl end-groups of PEG mediate the introduction of diverse functionalities capable of cross-linking; therefore, PEG is a suitable candidate for hydrogel formation. Additionally, PEG-based hydrogels are particularly attractive for biomedical applications owing to the biocompatibility, hydrophilicity, and antifouling properties of the polymer.[50]
Exploiting the advantages of PEG, dithiol terminated PEG macromonomers, PEG1.5k-dithiol and PEG4k-dithiol, were synthesized. The successful functionalization of the macromonomers was confirmed by ATR-FTIR and 1H NMR spectroscopies (see characterization details in Supporting Information, Figures S1–S3). For the network formation, the PEG-dithiol macromonomer was combined with TPA, which served as the cross-linker and the light-absorbing unit, at a 2:1 PEGn-dithiol:TPA mole ratio in THF, a good solvent for both comonomers. The step-growth reaction was initiated by the addition of a catalytic amount of sulfamic acid, which is a thermally stable, non-toxic compound that could be easily removed from the hydrogels by washing with water.[44] The reaction mixture was first heated to 55 °C to remove the excess THF, and then to 80 °C. Gelation of the mixture was observed visually within 15–20 min, while after 2 h of heating, a transparent solid material was obtained (the cross-linked network). Networks prepared using dithiol-terminated PEG of Mn = 1500 g mol−1 (Mw/Mn 1.04) and 4000 g mol−1 (Mw/Mn 1.09), are denoted below as PEG1.5k hydrogel and PEG4k hydrogel, respectively. To confirm the formation of the dithioacetal linkages and therefore the proposed gelation mechanism, solid-state 1H and 1H-13C HSQC NMR spectra of the hydrogels were recorded (Figures 1a and S4–S6) (see characterization details in SI). The ATR-FTIR spectra of the PEG4k and PEG1.5k networks were also recorded (Figure S7, blue lines), however, the presence of the C-S vibration bands at 533 cm−1 in the spectra of the PEG-dithiol macromonomers (black lines), prohibited the verification of the presence of the dithioacetal bonds in the polymer network spectra.
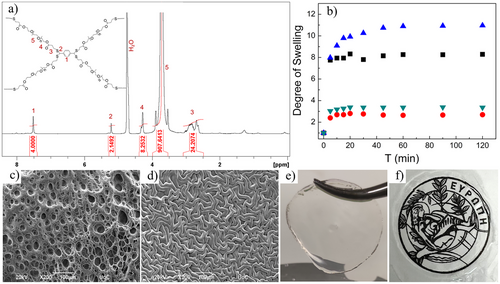
The swelling behavior of the prepared networks in water was examined at 20 and 37 °C (Figure 1b). The degrees of swelling of the hydrogels were found to depend on the Mn of the PEG and the solution temperature. The swelling ratio for the PEG1.5k hydrogel was lower both at 20 and 37 °C, due to the shorter PEG chains and thus the higher cross-link density of the hydrogels. A decrease in swelling was observed at 37 °C compared to 20 °C, which was more pronounced for the longer PEG chains and attributed to the thermo-responsive behavior of PEG in water. The morphology of freeze-dried hydrogels was examined by SEM (Figure 1c,d). Interestingly, the PEG4k hydrogel exhibited a highly porous structure, with pore sizes ranging from 5 to 40 µm (Figures 1c and S8). On the contrary, the PEG1.5k hydrogel demonstrated a rather dense structure resembling a wrinkled film (Figure 1d). These results are in good agreement with the lower degree of swelling for the PEG1.5k hydrogel. It is further noted that both hydrogels in their swollen state were soft and transparent (Figure 1e,f).
Rheological Characterization
The linear viscoelastic properties of the hydrogels were determined by monitoring the evolution of the storage (G’) and loss (G’’) moduli as a function of the applied stimulus. Dynamic frequency sweep (DFS) measurements were conducted in the linear viscoelastic regime at an angular frequency range 100–0.1 rad s−1, at a constant stress amplitude of 2 Pa, and at RT (298K). To validate the measurements, the DFS experiments were repeated three times for each type of hydrogel using three different batches (Figure S9). The PEG4k hydrogels exhibited a G’ plateau value of about 2.5 ± 0.38 kPa (Figure S9b), while for the PEG1.5k hydrogels, the DFS experiments showed a higher G’ of about 6.8 ± 2.1 kPa (Figure S9b), which correlated with the higher cross-link density of the latter sample. The measured G’ values for both samples are in good agreement with those reported earlier for similar PEG-based hydrogels.[36, 37, 51, 52] The small differences of the G’’ values were attributed to slight differences in the thickness of the hydrogels. The cross-link densities of the hydrogels were calculated using the Flory–Rehner and the rubber elasticity theory[53] for both ideal and non-ideal networks, and the calculated values are summarized in Table 1. The cross-link density refers to the number of cross-links per unit volume in a polymer network. As expected, the cross-link density of the PEG4k network was found to be approximately 3.5 times lower than the PEG1.5k network.
| Hydrogel | Plateau storage modulus (G’) (kPa) | Cross-link density (VcFR) (mol cm−3)a) |
Cross-link density (Vcrub) (mol cm−3)b) |
Relaxation time (τ) (s)c) | Photodegradation rate constant (kPD) (s−1)d) |
|---|---|---|---|---|---|
| PEG4k hydrogel | 2.5 ± 0.38 | 0.186 × 10−3 |
1.0 × 10−3 (0.5 × 10−3) |
τ1–4k 71.4 ± 6.5 τ2–4k 323.7 ± 15.5 |
kPD1-4k 14 × 10−3 kPD2-4k 3.08 × 10−3 |
| PEG1.5k hydrogel | 6.8 ± 2.1 | 2.37 × 10−3 |
3.79 × 10−3 (1.9 × 10−3) |
τ1–1.5k 1554.4 ± 33.6 τ2–1.5k 32 956.9 ± 9357.8 |
kPD1-1.5k 6.4 × 10−4 kPD2-1.5k 0.3 × 10−4 |
- a) Cross-link density calculated using the Flory-Rehner theory.
- b) Cross-link density calculated using the ideal rubber elasticity theory and, in parentheses, the non-ideal phantom network model[53] where cross-link junctions can fluctuate, assuming a cross-link functionality of 4.
- c) Relaxation rates and
- d) photodegradation rate constants determined by the normalized storage modulus versus irradiation time.
The photodegradation of the hydrogels was monitored by shear rheology using a quartz lower plate, which allowed the irradiation of the sample with UV light (λ = 254 nm, 0.063 mW cm−1), while the variation of G’ was monitored as a function of the irradiation time. It is worth mentioning that the photodegradation of the hydrogels is a process that occurs mainly on the surface of the hydrogel by photolyzing layer-by-layer the photosensitive moieties. So, the thickness of the gel, as well as the density of the photoactive sites, play a key role in the photodegradation rate of the system.[54] To study the viscoelastic changes of the hydrogels upon UV exposure, first, a PEG4k hydrogel with a thickness 350 ± 8.7 µm was placed between the plates of the rheometer and irradiated. Dynamic time sweep (DTS) experiments were conducted at a constant frequency of 10 rad s−1 and stress amplitude of 2 Pa, revealing a fast decay in G’ within the first 10 min of exposure, denoting the softening of the hydrogel because of the photocleavage of the labile dithioacetal bonds (Figure 2a, black squares). Indeed, G’ decreased from ∼2.5 kPa to ∼0.2 kPa after 10 min of irradiation, and then reached 0.01 kPa after 30 min of exposure. Interestingly, after about 40 min of irradiation, a crossover between G’ and G’’ was observed, indicating the gel-to-sol transition, and the disruption of the coherence of the network structure. To test the effect of hydrogel thickness, a PEG4k hydrogel with nearly half thickness ∼150 µm was prepared and irradiated under the same conditions. The DTS experiment revealed a faster gel-to-sol transition compared to the thicker (∼350 µm) hydrogel with the crossover at only ∼7 min of exposure (Figure 2a, blue rhombus). In the following, all measurements were carried out with a constant sample thickness of about 350 ± 8.7 µm. Similar changes in the shear modulus curve were observed for the PEG1.5k network upon photoirradiation (Figure S10). However, the irradiation time required for the disintegration of this hydrogel was substantially longer (∼8 h). This observation was associated with three critical considerations. The first is the higher cross-link density of the PEG1.5k hydrogel, which required a larger number of photons to photolyze the labile bonds.
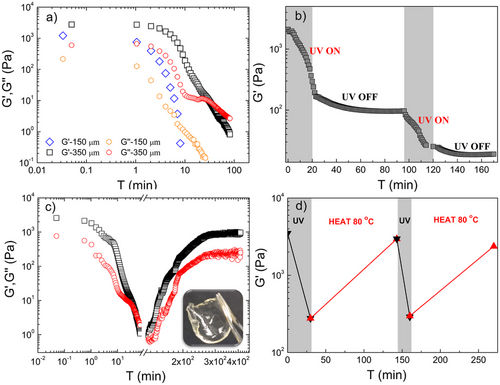
The second, is related to the partial absorption of the incident light by the aromatic photo-products which remain in the sample holder,[55] and the third is associated to smaller conformational entropy of the shorter chain, as proposed by Kowolik et al.[56] As shown in Figure S10, even after 8 h of irradiation, the crossover of the storage modulus G‘ and loss modulus G’ was not observed, signifying an incomplete transformation of the gel to the sol state, which was verified visually by the presence of a thin hydrogel layer upon lifting the rheometer's top plate.
Compared to hydrolytic or enzymatic cleavage, which are the main mechanisms employed for hydrogel degradation, photodegradation allows external spatiotemporal control of the applied light source, providing greater control over the hydrogel properties.[57-59] As a consequence, photodegradable hydrogels can be advantageous for various applications, including photoremovable wound dressings, photopatterning, regulation of the cellular functions (migration, proliferation, and differentiation), and controlled release of encapsulated cells or therapeutic agents.[16, 26, 37] We investigated the temporally controlled photodegradation of the PEG4k hydrogel (Figure 2b). Two irradiation cycles of 20 min duration each were applied to the hydrogel under shear. A rapid decrease in G’ from ∼2.5 to ∼0.16 kPa was found during the first irradiation interval (UV ON region), followed by a constant value of G’ for a period of 80 min when the irradiation ceased (UV OFF region). Upon switching on again the UV light (second UV ON region), the degradation recommenced and a fast drop in G’ to 0.025 kPa was observed, which again remained constant, when the light source was switched off (second UV OFF region). These results confirmed the ability to temporally control the cross-link density, hence the viscoelasticity of the hydrogel, and clearly show that the photolabile dithioacetal linkages within the polymer network can be easily cleaved upon light irradiation.
Owning to the high reactivity of thiols towards various functional groups (e.g., carbonyl, vinyl) under mild conditions, as well as their ability to undergo fast exchange reactions (dynamic exchange), we investigated the ability of the polymer networks to reform following photodegradation. Since the formation of the dithioacetal bonds required an elevated temperature, the reformation of the hydrogels after photocleavage was examined at 80 °C. The intrinsically low storage modulus of the PEG4k hydrogel in combination with its fast photodegradation rate and transformation into a sol, rendered this sample suitable to study the hydrogel reformation process and the recovery of its mechanical properties. Rheological analysis using DTS experiments was performed at 10 rad s−1 and 2 Pa on the PEG4k hydrogel with a thickness 350 ± 8.7 µm. The hydrogel was first irradiated with UV light for 70 min, when G’ gradually decreased from ∼2.2 to ∼0.07 kPa, and the crossover of G’ and G’’ was observed after ∼40 min of irradiation (Figure 2c). Then, the UV lamp was switched off, and the temperature in the rheometer was set to 80 °C. As shown in Figure 2c, the elastic modulus increased from ∼0.03 to ∼1 kPa within a period of ∼2.5 h and then it remained constant for ∼2 h. The increase of G’ and the moduli crossover indicated the sol-to-gel transition of the material and the formation of a polymer network with mechanical properties similar to those of the initial network, before photodegradation. Interestingly, upon completion of the test the reformed hydrogel appeared transparent, malleable and soft (Figure 2c, inset), similar to the original hydrogel, verifying the reformation of the network upon heating after photolysis. Based on these results, we suggest that the dithioacetal-based networks can easily photodegrade to a viscoelastic liquid state, while the degradation products can react again upon heating to reform the polymeric network. The photodegradation and the subsequent heating processes of the highly cross-linked PEG1.5k hydrogel revealed similar changes of the G’, G’’ curves to those found for the PEG4k hydrogel. However, as mentioned above, an extended irradiation time ∼8 h was necessary for photodegradation (Figure S10). Moreover, while G’ decreased from ∼6.5 to 0.016 kPa upon light exposure, its recovery during heating was only partial and reached ∼0.82 kPa after 3 h, while it remained constant for the next 8 h.
The reversibility of the dithioacetal network formation was examined in a 2-cycle irradiation-heating DTS experiment on the PEG4k hydrogel. Upon UV irradiation (30 min), G’ steeply decreased from ∼3.5 to ∼0.27 kPa and then increased to ∼3.0 kPa (85% recovery) with heating for ∼125 min (Figure 3d). Next, the sample was irradiated again for ∼10 min, which led to a rapid decay of G’ to ∼0.3 kPa, while upon heating (∼125 min), G’ reached a constant value of ∼2.4 kPa, signifying the reformation of the network (68% recovery) (Table S1). The slight decrease in modulus observed after each exposure cycle to the two stimuli was attributed to the formation of a small fraction of disulfide byproducts, which hindered the quantitative reformation of the polymer network (see “Insights into the photodegradation mechanism” section below). To prevent dehydration of the samples and minimize local fluctuations in water content during heating, the hydrogels were fully immersed in water. Any evaporated water was replenished in the rheometer bath surrounding the measuring fixture, ensuring uniform sample hydration.
The photodegradation kinetics of the system were determined by calculating the relaxation times (τ) from the normalized modulus (G’t/G’0) as a function of exposure time (Figure S11). The hydrogels exhibited two distinct τ values, τ1–4k = 71.4 ± 6.5 s and τ2–4k = 323.7 ± 15.5 s for the PEG4k hydrogel, and τ1–1.5k = 1554.4 ± 33.6 s and τ2–1.5k = 32 956.9 ± 9357.8 s for the PEG1.5k hydrogel (Table 1). These values signify that the photodegradation of the dithioacetal hydrogels is governed by two kinetic processes, one faster, which takes place during the initial exposure time, and a second at longer irradiation times. Dithioacetal bonds connected to stretched PEG strands require less energy to undergo photocleavage, hence these bonds are cleaved first, with photodegradation rate constants kPD 14 × 10−3 s−1 for the PEG4k hydrogel and 6.4 × 10−4 s−1 for the PEG1.5k hydrogel, while the rest of the cross-links, which are connected to more relaxed polymer strands, are cleaved at a second stage with kPD 3.08 × 10−3 s−1 and 0.3 × 10−4 s−1 for the PEG4k hydrogels and PEG1.5k hydrogels, respectively. The kPD values clearly show that the degradation kinetics of the PEG1.5k hydrogel is one order of magnitude slower compared to the PEG4k hydrogel. Interestingly, the G’t/G’0 of the thinner PEG4k hydrogel revealed one relaxation time (τ = 103.3 ± 3.1 s), signifying that the photodegradation process is governed by a single kinetic process. This result was attributed to the fewer structural inhomogeneities (e.g., loops) present in the thinner hydrogel sample, which resulted in fewer stretched PEG strands, and thus a single photo-degradation rate. It is noteworthy to mention that the dithioacetal networks presented herein undergo photocleavage with rates of the same order of magnitude as the extensively studied o-NB ester or coumarin-ester based photodegradable hydrogels.[27, 28, 36] However, the photodegradation of a material is directly related to the intensity of the light source, which in our case was significantly lower (0.063 mW cm−1) compared to that used to cleave the ester-based hydrogels (4–40 mW cm−2), revealing the high sensitivity of the S─O─S bond to light irradiation,[27, 31, 36] and therefore rendering these photosensitive materials promising candidates for photopatterning applications, network recycling and drug delivery technologies.
Photo-Controlled Release
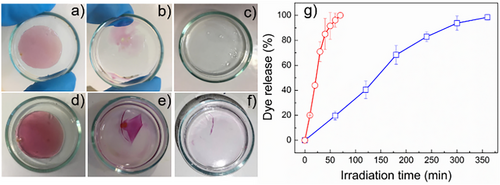
As indicated by the rheological measurements, the dithioacetal hydrogels can be effectively photodegraded at very low exposure intensities, whereas the photodegradation process can be remotely controlled by the use of the light source. These characteristics render our hydrogels particularly attractive for applications in 3D photopatterning, as smart photoremovable wound dressings or photodegradable and recyclable plastics. In all these applications, the encapsulation of active compounds such as antimicrobial agents, drugs or nanoparticles for catalysis (e.g., TiO2 for water purification) is highly desirable. To study the loading and releasing capacity of the dithioacetal hydrogels, a hydrophobic dye (Sudan Red, SR) was incorporated as a model compound within the porous structure during hydrogel formation and its release profile was investigated under light irradiation. As shown in Figure 3a,b, the photocleavage of the dithioacetal cross-links, upon irradiation, resulted in the disruption of the porous hydrogel structure and the gradual release of the dye. For both samples, the released dye exhibited an almost linear dependance with exposure time up to 80% release. The release profile of the dye molecules for the PEG4k hydrogel was significantly higher compared to the PEG1.5k hydrogel due to the faster photodegradation of the network. Specifically, 44% of the dye was released after 20 min of exposure of the PEG4k hydrogel (Figure 3c, red dots) while only ∼8% of the dye was released from the PEG1.5k hydrogel at the same irradiation time (Figure 3c, black squares). Notably, in contrast to the rheological study, the photo-decomposition of the PEG1.5k hydrogel was quantitative in this experiment due to the replacement of the aqueous medium by fresh water after each irradiation cycle, which also removed the aromatic photoproducts that absorb light and could possibly hinder the photodegradation process. It is important to note that the dye release rate from the hydrogels is influenced not only by the hydrogel's photodegradation rate but also by several other factors. These include the solubility of the dye molecules in water (with more hydrophilic molecules leaching out faster than less hydrophilic ones), the polymer-dye interactions, such as hydrogen bonding, π-π stacking, electrostatic forces, and others, and therefore a quantitative comparison of the photodegradation rate determined by rheology with the dye release rate would not be credible.
Insights into the Photodegradation Mechanism
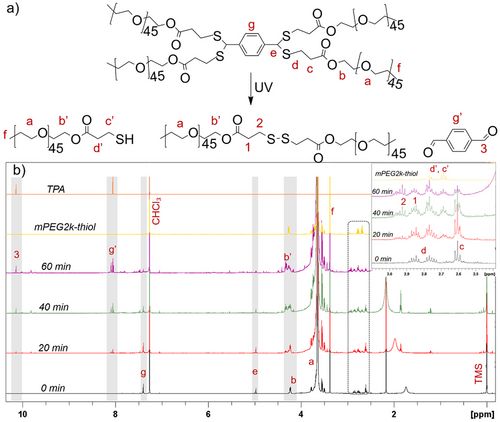
To further elucidate the reversible viscoelastic properties of the dithioacetal hydrogels, insights into the photodegradation/reformation mechanism were gained by studying the response to light irradiation of a small dithioacetal analogue. A 4-arm star polymer comprising a monofunctional thiolated PEG of molecular weight Mn = 2000 g mol−1 linked to the TPA molecule via dithioacetal bonds was synthesized and used for the photodegradation study (see supporting information and Figures S12–S14). The synthesis and the photodegradation kinetics of the star polymer were monitored by 1H NMR spectroscopy. D2O was selected as the solvent for the degradation study to provide similar environmental conditions to those used for the degradation of the swollen hydrogels. As shown in Figure S15, the signal at δ 5.22 ppm, attributed to the dithioacetal protons (He), decreased significantly after 20 min of exposure to light irradiation, and disappeared after 60 min of irradiation, indicating the photocleavage of the dithioacetal bonds. Concomitantly, the peak at δ 4.26 ppm, which corresponds to the methylene protons (Hb) next to the ester bonds of mPEG2k, was gradually shifted to 4.30 ppm (Hb’) denoting the cleavage of the mPEG chains from the star structure and the formation of the thiolated mPEG. Moreover, the signals at δ 2.60 and 3.03 ppm, which correspond to the methylene protons of the mercaptopropionate group, Hd and Hc, respectively, gradually decreased and four new peaks emerged in the δ 2.60 to 3.03 ppm range, signifying the formation of two photoproducts, the mPEG-thiol macromonomer and a small fraction of mPEG-S-S-mPEG dimer (Figure S16, inset). The latter explains the partial recovery of networks’ mechanical properties after photodegradation, since the dimers consume the thiol end groups of mPEG, preventing their reaction with the TPA molecules. 1H NMR spectroscopy revealed that the protons of the TPA molecule also underwent changes upon light irradiation. The peak at δ 7.53 ppm (Hg), attributed to the aromatic protons of the TPA ring, decreased with irradiation time, while several new peaks in the δ 7.4 and 8.5 ppm range appeared (Figures S15 and S16), denoting the cleavage of the dithioacetal bonds and the release of the aromatic molecules. However, due to the reduced solubility of the aromatic compounds in D2O, the verification of their structure was not possible. To confirm the formation of the aromatic photoproducts, the photodegradation experiment was studied in CDCl3 which solubilizes both comonomers (Figure 4). The decrease of the peak at δ 4.97 ppm attributed to the dithioacetal protons, confirmed the photocleavage of the labile cross-links. Irradiation induced the cleavage of the dithioacetal bonds and the release of the precursor mPEG2k-thiol polymer, as well as the mPEG-S-S-PEGm dimers. The appearance of a peak at δ 10.15 ppm, corresponding to the aldehyde protons of TPA (H3), confirmed the formation of the initial TPA comonomer.
Based on the above results, the proposed mechanism of photodegradation and re-formation of the dithioacetal cross-links of the polymer networks is illustrated in Scheme 1. Upon irradiation at very low intensity, the aromatic moieties of the TPA comonomer absorb the incident photons and, via an energy transfer process, cleave the labile C─S bonds located next to the aromatic groups, which have a very low bond energy (272 kJ mol−1), even lower than that of the C─O bonds (358 kJ mol−1) of the photosensitive polyacetals studied earlier by us.[60] Following the cleavage of the dithioacetal bonds in the presence of H2O molecules, the initial TPA comonomer and the thiol-terminated PEG chains are obtained as the main photoproducts, justifying the thermo-reversibility of the network structure, as well as a small fraction of PEG-S-S-PEG dimers, which have a minimum effect on the network reformation, as revealed by the rheological data.
Conclusion
The present work describes a versatile strategy for developing novel transparent, soft, stimuli-reversible hydrogels, which possess two distinct characteristics: the on-demand photodegradation and the thermo-induced reformation. It is remarkable that the introduction of the labile dithioacetal cross-links within the polymer network renders the material photodegradable at very low light intensities, but also thermo-reversible, under the action of two orthogonal stimuli, light irradiation and temperature. The hydrogels were synthesized via a facile addition reaction of a dialdehyde, TPA, and dithiol end-functionalized PEG chains under mild acidic conditions, yielding the formation of a dithioacetal cross-linked network. TPA was chosen as the photo-absorber moiety, placed next to the labile dithioacetal linkages, enabling and accelerating the photolysis process. By varying the length of the PEG strands in the network, the physicochemical and mechanical properties, including the cross-link density, porosity, storage modulus and photodegradation rate of the hydrogels were varied. Mechanistic studies on a dithioacetal star polymer analogue, revealed a chemical recycling process to the initial TPA monomer and the PEG-dithiol macromonomers as the main photoproducts after photolysis, enlightening the mechanism of network reformation upon heating the system at a mild temperature of 80 °C, as confirmed by rheology. The PEG4k hydrogel successfully underwent two cycles of photodegradation and reformation upon heating, restoring the initial mechanical properties of the hydrogel, and thus revealing the re-processability of the system. Finally, the novel hydrogels were tested in terms of their ability to capture and release, on-demand, hydrophobic molecules (e.g., drugs, antibiotics) upon light irradiation. The release of a hydrophobic dye, Sudan Red, from the PEG4k hydrogel was found to be ∼6 times faster compared to that of the PEG1.5k hydrogel, in qualitative agreement with the photodegradation rates of the respective hydrogels.
This proof-of-concept study of a new photodegradable and thermally reversible chemistry may pave the way for the generation of a novel class of materials with on demand photodegradable and thermo-reversible properties. By replacing the TPA cross-linker with other photo-absorbing moieties, the irradiation wavelength required for photodegradation can be conveniently shifted to higher wavelengths in the visible or even the NIR regime, while substitution of the PEG elastic strands of the network by other dithiol end-functional polymers, for instance polyurethanes or polydimethylsiloxanes could result in a variety of re-processable materials for diverse applications. In addition, the proposed chemistry could be employed in the biomedical field to bind drug molecules or proteins, which present abundant thiol functionalities, onto the polymer network structure. As a new addition in the library of photodegradable and thermo-reversible polymer networks, we anticipate that the proposed system, which relies on thiol and aldehyde functionalities, that are easily accessible in a wide range of commercial (macro)monomers will provide opportunities to develop functional materials for a broad array of applications in the biotechnological and sustainable development fields.
Supporting Information
The authors have cited additional references within the Supporting Information.
Acknowledgements
The authors would like to acknowledge Prof. Apostolos Spyros for assistance with the solid-state NMR facility and Mr. Stefanos Papadakis for his technical assistance with the SEM measurements.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




