Isoreticular Synthesis of Mesoporous Metal-Organic Polyhedra with Permanent Porosity to Gas and Water
Abstract
Synthesis of mesoporous metal-organic cages or polyhedra (MOCs or MOPs) that retain their porous functionality in the solid-state remains challenging, given their tendency to collapse upon desolvation. Herein, we report the use of the isoreticular expansion approach to synthesize two permanently porous Rh(II)-based octahedral MOPs within the mesoporous regime. Our mesoporous MOPs, featuring internal cavities of up to 12.5 nm3, withstand the activation process, enabling their use as solid-state adsorbents for gases and water. In particular, the largest mesoporous MOP, named BCN-17, captures up to 0.47 gwater·gMOP−1 and exhibits an S-shaped water-sorption isotherm with a hysteresis loop, characteristic of mesoporous materials.
Introduction
Synthetic chemists have long been inspired by Nature's unparalleled ability to create large, well-defined and functional molecular architectures. Seeking to replicate these remarkable structures,[1, 2] chemists have turned to supramolecular chemistry, the assembly of molecular building blocks into large molecules.[3, 4] Among the different types of large molecular architectures,[5] metal-organic cages (MOCs) in the mesoporous regime (cavity diameter > 2 nm) stand out due to their (macro)molecular host–guest chemistry. Mesoporous MOCs have typically been assembled either through the controlled assembly of numerous building blocks or the use of organic ligands with increasing sizes. In the first approach, the bent angle of the organic ligand is carefully adjusted to achieve topologies with numerous building blocks, such as the Goldberg polyhedron, which is assembled from 144 components and has a diameter of 5.9 nm.[6] In the second approach, known as isoreticular expansion and commonly employed for metal-organic frameworks,[7, 8] the size of a parent cage is enlarged by using ligands of increasing size but having the same bent angle, such that the final structure retains the initial geometry.[9, 8, 10, 11] Large MOCs assembled from either of these approaches are typically used in solution, in applications such as biomolecular storage, catalysis or separation.[12-17] However, fabricating robust, large MOCs that are permanently mesoporous in the solid state remains a challenge, given their tendency to collapse upon desolvation.[18] To overcome this challenge, the robustness of the cage backbone must be enhanced to withstand the capillary forces during desolvation and maintain the integrity of the empty cavity formed upon solvent removal. This principle is exemplified by permanently mesoporous organic cages, which, owing to strong covalent bonding, resist collapse.[19, 20]
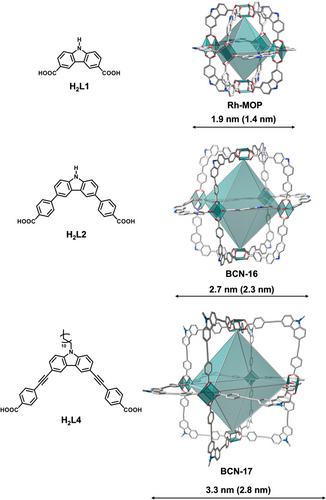
Herein, we demonstrate the synthesis of permanently porous MOCs with mesoporous cavities, highlighting that coordination bonds can stabilize large internal voids in discrete cages in the solid-state. To this end, we applied the isoreticular expansion approach to metal-organic polyhedra (MOPs) constructed from Rh(II) paddlewheel clusters (Figure 1). We hypothesized that the exceptional chemical and structural stability of these cluster units would enable the isoreticular expansion of the archetypal parent octahedral Rh(II)-based MOP, having the formula Rh12(L1)12 (where L1 is 9H-carbazole-3,6-dicarboxylate), while preserving its structural integrity upon desolvation.[21-23] This octahedral MOP, initially reported by Furukawa and coworkers and denoted as oct according to the Reticular Chemistry Structure Resource (RCSR) database,[24] features two opposite Rh(II) paddlewheel vertices separated by distance of 1.9 nm, and a microporous internal cavity of 1.4 nm3 (Figures S1–S10, Table S1).[25, 26] Building upon this octahedral MOP, we envisioned a double expansion of the carbazole ligand L1 to synthesize two new mesoporous isoreticular Rh(II)-based MOPs (hereafter named BCN-16 and BCN-17), featuring two opposite Rh(II) paddlewheel vertices separated by respective internal distances of 2.3 nm (BCN-16) or 2.8 nm (BCN-17), and respective internal cavities of 6.5 nm3 (BCN-16) or 12.5 nm3 (BCN-17) (Figure 1). We observed that, beyond control over the bite angle of the coordinating carboxylate groups in the carbazole ligands, precise tuning of the ligand twist-angle is also critical for synthesis of these MOPs. As we had hypothesized, the expanded MOPs retained their structural integrity upon desolvation, enabling their characterization as solid-state gas- and water-adsorbents. Interestingly, the largest mesoporous MOP, BCN-17, exhibited a stepwise water-adsorption isotherm characteristic of mesoporous materials and achieved a total water uptake of 0.47 g g⁻¹ (584.8 cm3 g⁻¹), the highest reported for discrete cages to date.
Results and Discussion
First Isoreticular Expansion of the Rh(II)-Based Octahedral MOP
To tackle the first isoreticular expansion of the parent Rh₁₂(L1)₁₂ MOP, we began with the synthesis of 4,4′-(9H-carbazole-3,6-diyl)dibenzoic acid (H2L2), an expanded version of L1 that adds a benzene ring between the carbazole core and the adjacent carboxylic acid group (Figures S11–S20). Once synthesized, H2L2 was reacted with Rh(II) acetate under solvothermal conditions. The complexation reaction yielded a green solution. The crude product was precipitated out with diethyl ether (Et2O), which afforded a green solid that was further washed with methanol (MeOH) to remove unreacted reagents and then, dried. Proton nuclear magnetic resonance (1H-NMR) analysis of the redissolved crude product in DMSO-d6 confirmed the success of the complexation reaction, as the aromatic signals appeared broad and shifted relative to the free ligand (Figure S21). Diffusion-ordered spectroscopy (DOSY) NMR analysis of the crude product revealed a diffusion coefficient of 5.1 · 10−11 m2 s−1, consistent with the synthesis of a large, discrete metal-organic assembly (Figure S22). Matrix-Assisted Laser Desorption/Ionization-Time-of-Flight (MALDI-TOF) mass spectroscopy of the crude product revealed that it contains at least two different metal-organic compounds, having the formulae [Rh10(L2)10 + H+]+ (found mass 5079.5, expected mass 5084.0 g mol−1) and [Rh12(L2)12 + H+]+ (found mass 6100.6, expected mass 6100.6 g mol−1) (Figure S23).
Isolation of supramolecular compounds through conventional separation techniques such as chromatography is difficult, due to their similar affinity to the stationary phase. Accordingly, we aimed to purify the crude mixture by recrystallization (Figure 2). To this end, we exposed a solution of the crude product in N,N-dimethylacetamide (DMA) to MeOH vapor, which yielded a microcrystalline material (A) and an amorphous solid (B). The crystalline material could be separated from the amorphous portion by sedimentation in MeOH, with the crystalline material A settling faster than did the amorphous fraction B. The crystalline product A was redissolved in DMA and recrystallized in the presence of 12 molecular equivalents of 4-tert-butylpyiridine and acetonitrile vapor. This crystallization yielded single crystals of sufficient quality for single-crystal X-ray diffraction (SCXRD) analysis (yield based on Rh(II): 2%). Note that subjecting the crude mixture to these crystallization conditions did not yield a crystalline material, indicating that the first purification step is required to obtain good quality single crystals. SCXRD analysis revealed formation of a peculiar trigonal prismatic MOP, which crystallizes in the orthorhombic space group Ibam with corresponding cell parameters a = 93.4627(6), b = 37.2998(4), c = 52.7312(3) Å (Table S2). This MOP (hereafter named BCN-13), which is denoted as trp according to the RCSR database, comprises six Rh(II) paddlewheel units and 12 L2 ligands (formula: [Rh12(L2)12]). Although a few examples of this type of structure have been reported,[27, 28] to the best of our knowledge, this is the first-ever report of one with metal-paddlewheel clusters. 1H-NMR, DOSY-NMR and Ultraviolet-visible (UV–vis) spectroscopy all confirmed the purity of BCN-13 and notably, the absence of free ligand (Figures S24–S26).
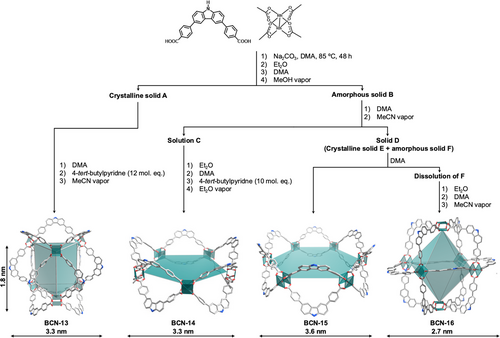
After obtaining pure BCN-13 from the microcrystalline A, we purified the amorphous portion B. Firstly, it was redissolved in DMA and then, exposed to acetonitrile vapor. This yielded a purple solution (C) and a solid (D). Addition of Et2O to C caused a solid to precipitate out, which was redissolved in N,N-dimethylformamide (DMF) and then, crystallized in the presence of 4-tert-butylpyridine and Et2O vapor to yield single crystals (yield based on Rh(II): 36%). SCXRD analysis revealed the formation of an unprecedented metal-organic polygon (hereafter named BCN-14), comprising a five-membered ring that crystallizes in the monoclinic space group P21/n with corresponding cell parameters a = 34.4336(3), b = 28.8627(3), c = 58.0490(3) Å, and α = γ = 90°, β = 93.1923(7)° (Table S3). BCN-14 comprises five Rh(II) paddlewheel clusters and 10 L2 ligands in a double-walled configuration (formula: [Rh10(L2)10]). Its purity was confirmed by 1H-NMR, DOSY-NMR, Fourier-transform infrared spectroscopy (FTIR), UV–vis, MALDI-TOF, thermogravimetric analysis (TGA) and powder X-ray diffraction (PXRD) (Figures S28–S34).
Having obtained crystalline BCN-14 from B, we next aimed to characterize D. Closer observation of D under optical microscope revealed that it comprises rectangular single crystals (E) and amorphous spheres (F) (Figure S36). When DMA was added to the solid, F dissolved but E remained (yield based on Rh(II): 20%). SCXRD analysis of the rectangular crystals E revealed formation of a second metal-organic polygon (hereafter named BCN-15), comprising a six-membered ring built up from six Rh(II) paddlewheel units and 12 double-walled L2 ligands (formula: [Rh12(L2)12]), which crystallizes in the monoclinic space group P21/c with corresponding cell parameters a = 31.4498(3), b = 39.6841(5), c = 40.5297(4) Å, and α = γ = 90°, β = 90.4263(8)° (Table S4). The purity of BCN-15 was confirmed by MALDI-TOF, FTIR, TGA, and PXRD (Figures S37–S40).
Finally, the DMA solution containing F was exposed to acetonitrile, which afforded rhombic single crystals (hereafter named BCN-16; yield based on Rh(II): 32%). Note that BCN-16 could only be crystallized in the presence of acetonitrile after all the other compounds had already been isolated. SCXRD analysis corroborated formation of the initially expected octahedral isoreticular MOP, having molecular formula of Rh12(L2)12. Structure analysis revealed that BCN-16 is isostructural to the parent microporous octahedral Rh12(L1)12 MOP, crystallizing in the triclinic space group P-1 with corresponding cell parameters a = 27.2945(8), b = 28.0465(6), c = 29.7602(6) Å, and α = 111.087(2)°, β = 112.027(2)° and γ = 101.813(2)° (Table S5). BCN-16 exhibits internal and external diameters of 2.3 and 2.7 nm (calculated from opposite paddlewheel units in the octahedron), and an internal cavity of 6.5 nm3 (Figure S51). The purity of BCN-16 was further confirmed by 1H-NMR, DOSY-NMR, FTIR, UV-vis, MALDI-TOF, TGA and PXRD (Figures S42–S48).
Geometry Mismatch in Discrete Metal-Organic Assemblies
Interestingly, the structures of BCN-13, BCN-15, and BCN-16 are isomers: each one comprises six rhodium paddlewheel units and 12 L2 ligands. However, they differ vastly by geometry, chiefly by the connectivity of their respective Rh(II) paddlewheel clusters. Paddlewheel metal centers are commonly understood to be 4-connected (4-c) clusters; therefore, we had expected this connectivity and indeed observed it in the octahedral cage BCN-16. In this structure, the L2 ligand is planar, yielding what it has been denoted as the “single-walled configuration” of the ligand (Figure 3a).[29, 30] Consequently, BCN-16 can be considered as the default structure for the L2 ligand in combination with a paddlewheel metal cluster. Conversely, BCN-15 adopts a geometrically mismatched structure, influenced by the twist of the phenyl groups surrounding the carbazole core, which renders the ligand non-planar (Figure 3b). In this arrangement, each paddlewheel behaves as a 2-connected (2-c) cluster due to the double-walled configuration of the ligands (Figure 3a). Although not isomeric to BCN-15, BCN-14 exhibits an analogous non-planar double-walled ligand configuration, forming a pentagonal metal-organic polygon with 2-c Rh(II) paddlewheel clusters. The Rh(II) paddlewheel cluster remains undistorted in both polygons, with the only difference being the twist angle of the L2 ligand, measured at 56.3 ± 5.8° for BCN-14 and 58.2 ± 6.5° for BCN-15 (Figures S35 and S41). Therefore, we attributed the formation of both polygons to slight variations in the twist angle of the L2 ligand. These small differences in ligand conformation are likely close in energy, allowing both polygons to form under the tested reaction conditions.
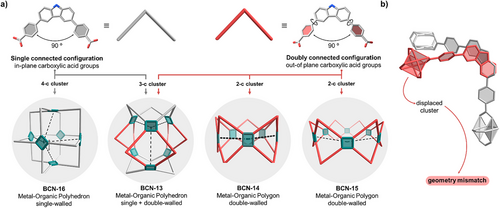
The structure of BCN-13 arises from the combination of planar and non-planar arrangements of L2, leading to the uncommon 3-connected (3-c) Rh(II) paddlewheel cluster (Figure 3a). Specifically, the 3-c Rh(II) paddlewheel clusters result from the double-walled configuration of the ligands, which account for the edges of each lateral rectangular face, whereas only single-walled ligands compose both triangular bases of the structure.[31-33] As with the structures analyzed above, the Rh(II) paddlewheel cluster in this case remains undistorted, with the final structure being attributed to the twist of the ligand, which is 55.1 ± 9.2° for the ligands with double-walled configuration and 14.4 ± 6.2° for those with single-walled configuration (Figure S27). Overall, these results highlight that the twist angle between the carbazole and the adjacent benzene ring is as important as their bite angle in determining the structural outcome of the assembly reaction, in line with previous reports showing that subtle variations in ligand conformation -particularly twist angles- can direct the formation of distinct cage topologies.[3]
Second Isoreticular Expansion of the Rh(II)-Based Octahedral MOP
Geometrical analysis of the structures formed with H2L2 revealed that the final structure depends not only on the ligand's bite angle but also on the twist of the linker. Notably, the octahedral cage formed only when the ligand had adopted a planar conformation. Guided by this observation, we designed the ligand for the second isoreticular expansion to induce planarity between the carbazole core, the benzene ring, and the carboxylic acid groups. To this end, an alkyne bond was introduced between the phenyl and carbazole moieties to enable synthesis of 4,4′-((9H-carbazole-3,6-diyl)bis(ethyne-2,1-diyl))dibenzoic acid (H2L3) (Figures S53–S62). However, all solvothermal reactions performed with H2L3 and Rh(II) acetate under solvothermal conditions resulted in insoluble amorphous precipitates that could not be characterized.
We then explored strategies to increase the solubility of the ligand and avoid early kinetic traps in the form of amorphous solids during the complexation reaction. To this end, an alkane chain was added to the carbazole core of the ligand to enable synthesis of 4,4′-((9-dodecyl-carbazole-3,6-diyl)bis(ethyne-2,1-diyl))dibenzoic acid (H2L4) (Figures S63–S72), which was then reacted with Rh(II) acetate under solvothermal conditions to yield green rectangular crystals in good yield (68%). These crystallize in the triclinic space group P-1 with corresponding cell parameters a = 30.8769(2), b = 33.8701(3), c = 36.1112(2) Å, and α = 107.5252(6)°, β = 100.1424(6)° and γ = 114.2237(6)° (Table S6). SCXRD analysis corroborated formation of the expected octahedral cage (hereafter named BCN-17), isostructural to the parent microporous octahedral Rh-MOP and BCN-16. Remarkably, BCN-17 possesses internal and external diameters of 2.8 and 3.3 nm (calculated from opposite paddlewheel units in the octahedron), and an internal cavity of 12.5 nm3 (Figure S79), which represents the biggest cavity reported for a paddlewheel-based MOP (Table S7). The purity of BCN-17 was confirmed by MALDI-TOF, FTIR, TGA, and PXRD (Figures S73–S76).
Solid-State Sorption Properties of Isoreticular Octahedral Rh-MOPs
To evaluate the solid-state adsorption properties of the synthesized octahedral MOPs, we conducted gas- and vapor-adsorption studies on BCN-16, BCN-17 and their parent microporous octahedral Rh-MOP, and compared their respective values. To this end, all three MOPs were initially fully evacuated by first exchanging them with MeOH and then, drying them with supercritical CO2. This resulted in the amorphization of BCN-16 and BCN-17, and a partial loss of crystallinity in the parent microporous octahedral Rh-MOP (Figures S8, S49 and S77). However, the molecular structure of all tested MOPs was preserved in all cases, as confirmed by MALDI-TOF analysis of the activated samples (Figures S9, S50 and S78).
Next, we ran N2-sorption experiments at 77 K on all three MOPs, which demonstrated their permanent porosity, as evidenced by their corresponding Brunauer–Emmett–Teller (BET) surface areas: 812 m2 g⁻¹ for the parent Rh-MOP; 780 m2 g⁻¹ for BCN-16; and 121 m2 g⁻¹ for BCN-17 (Figure 4a,b). The parent microporous octahedral Rh-MOP exhibited the expected type I isotherm, whereas BCN-16 and BCN-17 exhibited type IV isotherms, as typically observed in mesoporous materials (Figures S81–S89).[34] We attributed the slight decrease in the BET surface area after the first isoreticular expansion to the higher molecular weight of BCN-16 than of the parent microporous Rh-MOP. However, when the isotherm is normalized per mol of MOP, BCN-16 outperforms the parent microporous Rh-MOP (87.2 mol N2 mol−1 BCN-16 versus 49.5 mol N2 mol−1 Rh-MOP at 1 bar). In contrast, we attributed the lower BET surface area and maximum uptake (36.4 mol N2 mol−1 MOP at 1 bar) of BCN-17 to its large cavity-volume-to-surface ratio and to the presence of its surface alkane chains, which can block gas diffusion pathways to the inner pore. The detrimental effect of bulky surface groups on the solid-state adsorption properties of MOPs has been previously reported.[35, 36] The permanent porosity of all synthesized Rh-MOPs was further confirmed through CO2-adsorption measurements run at 200 K, which revealed a similar trend to the N2-adsorption experiments. BCN-16 exhibited the greatest adsorption, with an uptake of 80 mol CO2 mol−1 MOP at 1 bar, which we attributed to its large, accessible cavity (Figure 4c, Figures S90–S92).
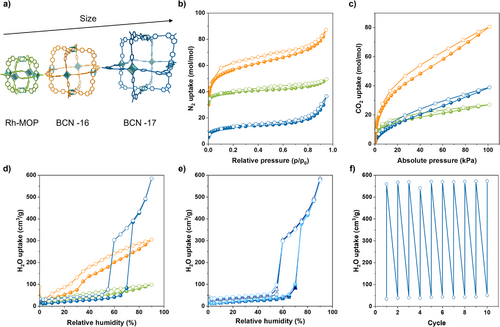
Next, we investigated the water-adsorption properties of the three Rh-MOPs. To this end, we ran water-adsorption isotherms at 298 K, in which the final water uptake at 90% relative humidity increased with cavity size, reaching 0.08 g g−1 (98.2 cm3 g⁻¹, 18.7 mol H2O mol−1 MOP) for the parent Rh-MOP; 0.24 g g−1 (304.4 cm3 g⁻¹, 82.9 mol H2O mol−1 MOP) for BCN-16; and 0.47 g g−1 (584.8 cm3 g⁻¹, 225.7 mol H2O mol−1 MOP) for BCN-17 (Figure 4d, Figures S93–S95). The water uptake value for BCN-17 represents the highest value reported for a cage[37] and is comparable to values for other absorbent materials such as metal-organic frameworks or covalent-organic frameworks.[38, 39] A deeper analysis of the water-adsorption isotherms revealed that the parent microporous octahedral Rh-MOP exhibits a reversible isotherm without a step and a moderate final uptake, consistent with the pore-filling mechanism observed in microporous hydrophilic materials, such as zeolites and MOFs.[40] Conversely, the isotherms (type V) for both mesoporous BCN-16 and BCN-17 feature a step that indicates the occurrence of capillary condensation, characteristic of adsorbents with pore sizes exceeding 2 nm.[41, 42] For BCN-16, the step of the “S”-shaped isotherm occurs at α = 0.45 (where α is the relative humidity at 50% uptake). More remarkably, in the case of BCN-17, the water adsorption is entirely dominated by capillary condensation exhibiting an “S”-shaped isotherm that resembles that of mesoporous materials such as MCM-41 and MIL-101.[43, 44] We ascribed the high relative humidity at which the step occurs in the isotherm of BCN-17 (α = 0.57) to the hydrophobicity of BCN-17, imparted by its surface alkyl chains. The hydrophobic character of BCN-17 was further confirmed by contact-angle measurements, which revealed a value of 88.83 ± 0.71° (Figure S80), in contrast to BCN-16, which showed complete wetting with a contact angle of 0° (Figure S52). These findings are consistent with observations in mesoporous MOFs, where total uptake is mainly governed by the mesopore size, while the position of the adsorption step is primarily dictated by the hydrophilic or hydrophobic nature of the framework.[45, 46]
Finally, we sought to evaluate the potential of BCN-17 as a water adsorbent. In addition to high water-uptake and stepwise adsorption at ambient pressure, a good water adsorbent must also exhibit cyclability and facile regeneration.[39] Thus, we subjected BCN-17 to three consecutive adsorption–desorption water isotherms, which did not reveal any significant differences between cycles (Figure 4e). Moreover, to assess its regeneration and recyclability, BCN-17 was exposed to alternating high (90%) and low (20%) relative humidity (Figure 4f), which confirmed that it can maintain its performance over 10 cycles. Overall, the performance of BCN-17, characterized by high water-uptake, an S-shaped isotherm, and ease of regeneration and recyclability, meets the key requirements for materials used in humidity control.[47]
Conclusion
In summary, we have synthesized a series of isoreticularly expanded, Rh-based, octahedral MOPs exhibiting internal cavities within the mesoporous regime. We have demonstrated that linker planarity is crucial for developing novel finite structures, as evidenced in the synthesis of the first expanded MOP, BCN-16, in which three additional finite structures – a trigonal prismatic MOP, a hexagonal polygon, and a pentagonal polygon – are simultaneously assembled. We attributed formation of these additional structures to the torsional flexibility of the linker L2. Furthermore, since two of these additional finite structures are isomeric species of BCN-16, our results highlight the importance of characterizing products by PXRD together with MALDI-TOF to confirm the purity of any newly synthesized cage or MOP before its practical use. Finally, gas- and vapor-sorption studies revealed that the enlarged octahedral Rh-MOPs withstand the desolvation process, making them viable mesoporous materials for solid-state adsorption applications. In fact, the larger Rh-MOP, BCN-17, exhibited high water uptake (0.47 gwater·gMOP−1), accompanied by an “S”-shaped water sorption isotherm with a hysteresis loop. We are confident that our new processable materials will open avenues in water sorption-based applications.
Acknowledgements
This work has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement no. 101019003, grant refs. PID2021-124804NB-I00 funded by MCIN/AEI/10.13039/501100011033/ and by “ERDF A way of making Europe”, and the Catalan AGAUR (project 2021 SGR 00458). It was also funded by the CERCA Programme/Generalitat de Catalunya. ICN2 is supported by the Severo Ochoa Centres of Excellence program, Grant CEX2021-001214-S, funded by MCIN/AEI/10.13039.501100011033. A.C.S. is indebted to the Ramón y Cajal Program (RYC2020-029749-I Fellowship). C.v.B. thanks the Austrian Science Fund (FWF), Erwin Schrödinger fellowship for supporting the project J 4637. Y.Y. acknowledges the China Scholarship Council for scholarship support. M.S.G acknowledges the CONACyT for scholarship support.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.




