Highly Efficient and Selective Photocatalytic CO2 Reduction to Ethanol by Cu4 Clusters with Adjacent Cu Single Atoms Anchored on Carbon Nitride
Abstract
An efficient photocatalyst for CO2-to-CH3CH2OH conversion comprising atomically dispersed Cu clusters (Cu4) and single Cu sites coordinated with 2 N and 1 O anchored on two-step calcinated C3N4 (Cu4/Cu1@CN) is reported. It was found to catalyze the reduction of CO2 to ethanol with production rate of 154 µmol g−1 h−1 and selectivity of 98%, the yield rate and selectivity exceed most of the reported results for ethanol production. Experimental and theoretical calculations indicate that the high efficiency of this catalyst arises from the strong synergy between Cu4 clusters and adjacent Cu single atoms, which facilitates OC─CO coupling and promotes ethanol production.
Introduction
Reducing CO2 to various fuels by solar energy is a promising way to solve environmental problems and energy crisis caused by excessive CO2 emission from burning of fossil fuels.[1] C1 products, such as CO and HCOOH, have been commonly produced from catalytic CO2 reduction, whereas C2+ products with higher economic value and energy density are less reported.[2, 3] Ethanol is a versatile fuel that can be easily transported, and it has been widely used in food chemistry, chemical synthesis, and fuel cells.[4] However, there are few reports on the conversion of CO2 to ethanol via photocatalysis.
It is generally accepted that C2+ products generated from CO2 reduction starts from the deoxygenation of CO2 to form *CO, which will couple with neighboring *CO to form the crucial intermediate *CO─CO, followed by sequential multielectron and multiproton transfer to yield the corresponding C2+ products, such as ethanol.[5] The intrinsic difficulty in designing catalysts with active sites that can deoxygenate CO2 efficiently and at the same time be able to adsorb the CO produced for coupling with an adjacent adsorbed or free CO have limited the generation efficiency and selectivity of C2+ products.[6]
Single-atom catalysts (SACs) featuring well-defined single atom active sites have become an excellent platform for photo- and electrocatalytic CO2 reduction reaction (CO2RR) in recent years.[7-9] A number of SACs can efficiently activate CO2 molecules owing to their unique electronic and structural properties arising from low coordinated active sites and high surface energy.[10, 11] Among SACs, Cu-based materials are the only known catalysts that have shown high CO2RR activity for generating ethanol under mild conditions in electrocatalysis,[12-15] but the efficiency and selectivity in photocatalysis are in general low. For example, Cu single atoms on a UiO-66-NH2 support (Cu SAs/UiO-66-NH2) could catalyze CO2 to ethanol conversion at 4.22 µmol g−1 h−1 rate and 54.3% electron-selectivity.[16] An atomically dispersed indium and copper diatomic catalyst anchored on polymeric carbon nitride (InCu/PCN) catalyzes CO2 to ethanol conversion with the production rate of 28.5 µmol h−1 g−1 and selectivity of 92%.[17] Recently, Cu single-atom catalyst supported on carbon nitride featuring defected low-coordination Cu-N2 motif (Cu─N2─V) was reported to catalyze the reduction of CO2 to ethanol with 69.8 µmol g−1 h−1 rate and 97.8% electron selectivity.[18]
Considering that the coupling of *CO and/ or *CHO needs at least two active sites, adjacent single atoms that are close enough may be required to facilitate *CO → *CHO and CO─CO coupling.[19, 20] Indeed, in electrocatalysis, Cu-based SACs with efficient CO2 to ethanol conversion have been shown to change to Cu clusters during electrolysis, which are the real catalysts for CO2 to ethanol.[14, 21] Recently, Wang and Li[22] revealed by density functional theory (DFT) calculations that generation of C2+ products from CO2 reduction should require at least three adjacent Cu atoms. Thus, the rational design of a catalyst composed of Cu clusters may be a promising strategy to convert CO2 to ethanol or other C2+ products with high efficiency and selectivity via photocatalysis. Herein, we developed a catalyst composed of Cu4 clusters and adjacent Cu single atoms anchored on carbon nitride (Cu4/Cu1@CN). The number of Cu atoms in each cluster can be regulated by varying the concentration of the Cu precursor. We found that Cu4/Cu1@CN is a highly efficient CO2 to ethanol photocatalyst with production rate up to 154 µmol h−1 g−1 and selectivity of 98%, which is better than most reported photocatalysts for this conversion. The excellent light absorption and charge separation properties of Cu4/Cu1@CN contribute to its catalytic efficiency. Based on our experimental results and DFT calculations, the high efficiency of this catalytic system arises from synergistic effect between Cu4 cluster and Cu single atoms, resulting in lowering the C─C coupling energy barrier and enhancing the production of ethanol.
Results and Discussion
Catalyst Preparation and Characterization
Catalysts were prepared by a two-step calcination process with acidified g-C3N4 (a-C3N4) and Cu(NO3)2·3H2O as precursors, as illustrated in Figure 1a. Acidified g-C3N4 was obtained by exfoliating bulk g-C3N4 in concentrated H2SO4. Detailed synthesis steps are provided in Supporting Information. It has been reported that acid treatment could induce the generation of hydroxyl groups on the surface of bulk g-C3N4,[23] which is also confirmed by our FT-IR analysis. As shown in Figure S1, compared with primary bulk g-C3N4, new peaks appeared in the FT-IR spectra of a-C3N4 at 1536, 1194, and 1130 cm−1, which correspond to the stretching vibration of OH, ─NO, and ─CO, respectively, indicating the presence of these functional groups in a-C3N4. Hydroxyl groups contribute to the transformation of bulk C3N4 into a thin-layer structure, increasing the specific surface area.[23] Meanwhile, the hydroxyl groups can also coordinate with metal ions, which helps to anchor more metal ions and enhance the metal loading amount. Cu4/Cu1@CN was obtained when the mass ratio of Cu(NO3)2 to a-C3N4 was 30%, whereas Cu1@CN was obtained when the ratio was 3%. Acidified g-C3N4, calcined under the same conditions, was used as control sample and named as CN. The mass percentage of Cu in Cu4/Cu1@CN and Cu1@CN were determined to be 19.0% and 0.14%, respectively, by inductively coupled plasma mass spectrometry (ICP-MS).
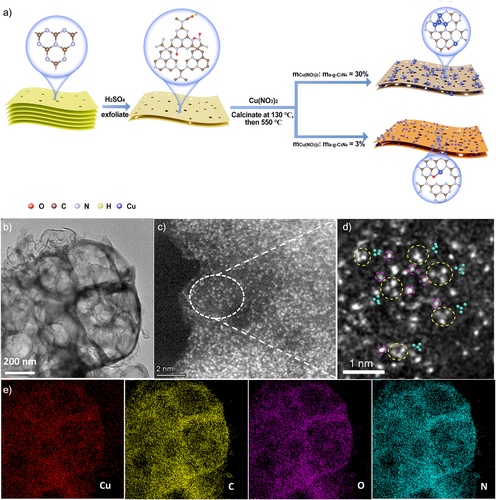
The morphologies of the samples were investigated by high resolution scanning electron microscopy (HRSEM) and transmission electron microscopy (HRTEM). All three samples show a porous layered structure; the number of pores increase with the increase of Cu content (Figures 1b and S2–S4). BET analysis shows that Cu4/Cu1@CN has the largest specific surface area (71.82 m2 g−1) (Figure S5 and Table S1); such porous structure with large specific surface area could provide more reactive sites and enhance light harvesting as well as mass transfer during catalysis.[24, 25] No particles are found in HRTEM images of Cu4/Cu1@CN and Cu1@CN. The energy-dispersive X-ray spectroscopy (EDS) mapping image shows that Cu, C, N, and O are uniformly dispersed in these two catalysts. The power X-ray diffraction (XRD) patterns of the three samples consist of one strong diffraction peak at 27.4° and one weak peak at 13.0°, corresponding to the orderly stacked (002) interlayer-plane and (100) in-plane ordering of tri-s-triazine units, respectively(Figure S6).[26, 27] Notably, the higher the amount of Cu used, the weaker is the peak at 13.0°, indicating that Cu loading disrupts the tri-s-triazine structure, leading to poor in-plane ordering. There are no peaks corresponding to Cu metal or other Cu compound in XRD, indicating the absence of Cu-based nanoparticles in Cu4/Cu1@CN and Cu1@CN. The atomic-resolution aberration-corrected HAADF-STEM images of Cu4/Cu1@CN show that the density of Cu atoms on the majority of the support is remarkably high (Figure 1c), primarily attributable to the ultrahigh Cu loading, as determined by ICP-MS. In the relatively thinner regions with lower Cu content, both isolated Cu single atoms and clusters are present. Upon magnification, it is evident that most of the clusters are formed by the close-packed 4 Cu atoms (Figure 1d). The distance between adjacent Cu atoms within these clusters ranges from 1.5 to 2.3 Å (Figure S7), signifying the formation of Cu─Cu bonds. Notably, it seems that there is always a Cu single atom next to a Cu4 cluster. Elemental mapping analysis shows that Cu, C, O, and N are uniformly dispersed in the sample (Figure 1e). On the other hand, Cu1@CN consists of many Cu single atoms (orange circles), as shown in Figure S8.
X-ray absorption fine structure (XAFS) analysis was used to determine the coordination environment and local structure of copper in Cu4/Cu1@CN and Cu1@CN. Figure 2a presents the Cu K-edge X-ray absorption near-edge structure (XANES) spectra of the two catalysts as well as Cu foil, copper phthalocyanine (CuPc), and CuO as references. Notably, the near-edge absorption peaks of Cu4/Cu1@CN and Cu1@CN are located between that of Cu foil and CuO, indicating that the Cu atoms in Cu4/Cu1@CN and Cu1@CN carry positive charges with oxidation state between 0 and +2. Figure 2b shows the Fourier-transformed extended X-ray absorption fine structure (EXAFS) spectra of these samples. The R space positions (prior to phase correction) of the Cu─Cu peak in copper foil is at 2.22 Å, the Cu─O scattering in the first shell of CuO and Cu─N scattering in the first shell of CuPc are at the R-space distances of 1.53 and 1.50 Å, respectively. Thus, the peak at 1.52 and 2.14 Å in Cu4/Cu1@CN are attributed to Cu─N/O and Cu─Cu. This is also confirmed by the wavelet transform contour plots of Cu K-edge weighted EXAFS as shown in Figure 2d. Figures 2c and S9a, as well as Table S2, show the corresponding EXAFS fitting results, which reveal that each Cu atom coordinates with 2 N atoms with bond length of 1.95 Å, 1 O atom with bond length of 1.80 Å, and 3 Cu atoms with bond length of 2.56 Å, demonstrating the coexistence of Cu4 cluster, Cu─N2, and Cu─O in Cu4/Cu1@CN.
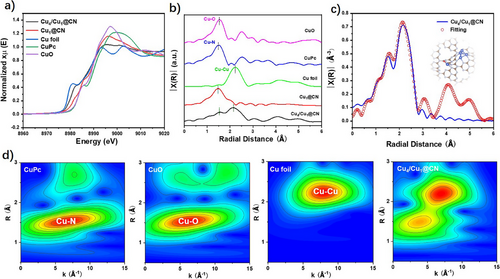
Similarly, the peaks at 1.47 Å in Cu1@CN is attributed to Cu─N/O. The fitting results show that one Cu atom coordinates with 2 N and 1 O atom, with the bond length of 1.96 and 1.81 Å, respectively (Figure S9b,c and Table S2). Combining the result of HAADF-STEM and wavelet transform contour plot (Figure S9d), it can be deduced that Cu single atoms with Cu─N2 and Cu─O coordination environment exist in Cu1@CN.
Based on the XAFS results and HAADF-STEM images, density functional theory (DFT) calculations were conducted to model the Cu coordination in Cu4/Cu1@CN and Cu1@CN. The optimized structures are inserted in Figures 2c and S9b, respectively. Cu4/Cu1@CN consists of Cu4 clusters anchored on CN via three Cu─N2 bonds and the adjacent Cu single atom is coordinated with 2 N and 1 O atoms. Cu1@CN consists of isolated Cu single atoms anchored on CN via Cu─N2 and Cu─O bonds.
X-ray photoelectron spectroscopy (XPS) was used to reveal the chemical nature of Cu4/Cu1@CN and Cu1@CN. Figure S10a shows the Cu 2p spectrum of Cu4/Cu1@CN; the main characteristic peaks appear at 932.9 and 952.5 eV, corresponding to Cu+ or Cu0 2p3/2 and 2p1/2, respectively. (Note that the peaks of Cu0 and Cu+ are located at similar positions in the XPS spectrum.)[28] The oxidation states of Cu have been further confirmed by Auger electron spectroscopy (AES) (Figure S10b). There are two deconvolved peaks of Cu+ (916.5 eV) and Cu0 (918.5 eV). Thus, the XPS analysis is consistent with XAFS result. On the other hand, XPS as well as the corresponding AES are unable to explicitly determine the oxidation states of Cu in Cu1@CN due to the relatively low signal-to-noise ratio associated with the low copper content.[29]
The N 1s XPS spectra were also fitted to compare the coordination environments of the catalysts (Figure S10c). The N 1s XPS spectra exhibit a broad feature with binding energy ranging from 397 to 402 eV. For Cu4/Cu1@CN, this feature can be deconvoluted into three peaks centered at 398.4, 398.9, and 401.02 eV, which can be assigned to pyridinic (C─N═C), tertiary (─N < or M─NC2), and pyrrolic (─NH) nitrogen, respectively.[29-31] Compared with CN, Cu4/Cu1@CN exhibits a much more pronounced feature associated with the tri-coordinated N (M─NC2), consistent with the expected Cu─N coordination. On the other hand, there is no obvious difference in N 1s XPS spectra between Cu1@CN and CN, which should be attributed to the low Cu content in Cu1@CN. Moreover, the additional peak centered at 404.4 eV for the three samples should be ascribed to terminal nitrate groups, charging effects, or π excitations.[32-34] The high-resolution O 1s spectra of Cu4/Cu1@CN (Figure S9d) can also be deconvoluted into three peaks centered at 530.6, 531.7, and 532.7 eV, corresponding to Cu─O, vacancy O, and adsorbed O, respectively.[35, 36]
Photocatalytic CO2 Reduction
Photocatalytic CO2 reduction was conducted in a solid–gas reaction system under irradiation with Xenon lamp. CO2 saturated with water was purged into the reactor for 30 min before irradiation. Detailed experimental procedures are provided in Supporting Information. The products formed from Cu4/Cu1@CN, Cu1@CN, and CN and their production rates are presented in Figures 3 and S11–13, as well as Table S3. Among the three catalysts, Cu4/Cu1@CN displayed significantly higher activity and selectivity for CH3CH2OH formation. Ethanol production increased linearly with time with rate of 154 µmol g−1 h−1 and selectivity of 98% (Figures 3a and S13); such performance is among the best in terms of both ethanol production rate and selectivity, compared with the reported CO2 to ethanol photocatalysts (Figure 3b).[16, 18, 37-42] Traces of CH3OH, CH4 and CO were also detected. Notably, the yield and selectivity of ethanol were almost unchanged in four consecutive cycles (Figure 3d). Additionally, TEM image, XRD patterns, and Cu 2p XPS spectra were almost the same as before the reaction (Figures S14–16), demonstrating the profound catalytic stability of Cu4/Cu1@CN. In contrast, Cu1@CN had the highest activity for CO and negligible activity to form ethanol. For CN, no ethanol was produced, only trace amounts of CH4 and CO were detected. The experiment with Cu4/Cu1@CN as catalyst under Ar also gave negligible products (Table S3). The apparent quantum efficiency for ethanol production was determined to be 0.012% after irradiating at monochromatic light of 350 nm for 20 h.
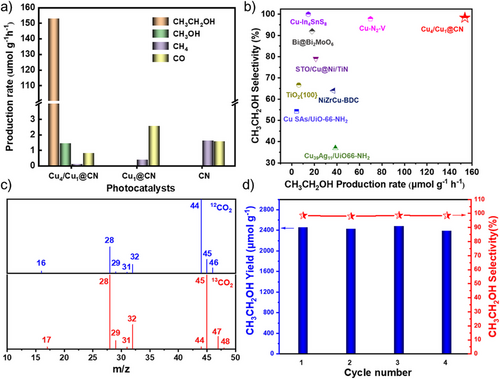
To verify the origin of ethanol, isotopic labeling experiments with 13CO2 were performed and the GC-FID spectra of gaseous products are shown in Figure S17. GCMS of gaseous products from use of 12CO2 show peaks at m/z = 16, 29, 31, 44, 45, and 46 in the mass spectrum, which are attributed to 12CH4, 12CH312CH2, 12CH2OH, 12CO2, 12CH312CH2O, and 12CH312CH2OH, respectively (Figure 3c). The peak at m/z = 28 should be attributed to N2 and 12CO. When 13CO2 was used, the peaks at m/z = 17, 29, 31, 45, 47, and 48 in the mass spectrum are assigned to 13CH4, 13CO, 13CH313CH2, 13CO2, 13CH313CH2O, and 13CH313CH2OH. The concentration of methanol in the gas phase product was extremely low, such that no corresponding peaks were detected in the GCMS analysis. The above results indicate that ethanol was produced from CO2 reduction.
As shown in Figure S18 and Table S4, O2 with the yield rate of 449 µmol g−1 h−1 was generated in the system using Cu4/Cu1@CN, which is equivalent to 1795 µmol g−1 h−1 electrons and H+ based on the reaction of water oxidation to O2. This is nearly equal to the mole of electrons and H+ consumed to produce 154 µmol g−1 h−1 ethanol from CO2 reduction (1844 µmol g−1 h−1), demonstrating that the holes generated by light irradiation were used to oxidize water to O2 and provide H+ for CO2 reduction.
Reaction Mechanism
The light adsorption property and charge separation efficiency of the catalysts were investigated by spectroscopic methods. The UV–visible diffuse reflectance spectra (UV–vis DRS) (Figure S19) of Cu1@CN and CN show a light absorption band from 300 to 700 nm, whereas Cu4/Cu1@CN exhibits a broader and enhanced light absorption from 300 to 800 nm. According to the Tauc plots (inset in Figure S19), Cu4/Cu1@CN has the narrowest bandgap (2.14 eV) compared with Cu1@ CN (2.68 eV) and CN (2.71 eV).
In addition, Mott–Schottky plots were employed to determine the conduction bands of the catalysts. The valence band of samples was calculated using the formula Eg = EVB − ECB. As shown in Figures S20 and S21, the band structures of all catalysts indicate their capability of oxidizing H2O to O2 and reducing CO2 to CO, CH4, CH3OH, and C2H5OH.
Transient photocurrent responses (Figure S22a) and electrochemical impedance spectroscopy (EIS) Nyquist plots (Figure S22b) were conducted to explore the separation of the photogenerated carriers. Cu4/Cu1@CN (1.52 µA cm−2) exhibited the highest photocurrent density in comparison to Cu1@ CN (0.15 µA cm−2) and CN (0.02 µA cm−2), and EIS Nyquist plots show that Cu4/Cu1@CN has the smallest semicircle radius, indicating the lowest transfer impedance. These results show that Cu4/Cu1@CN exhibits the optimal effect on the separation of photogenerated carriers. Steady-state photoluminescence (PL) spectra (Figure S22c) and time-resolved photoluminescence (TRPL) spectra (Figure S22d) further reveal the charge transfer dynamics of the catalysts. According to the intensity of the emission peak, Cu4/Cu1@CN has the weakest signal peak in the entire wavelength range compared with the other two catalysts, meaning that the recombination of photoinduced carriers could be effectively inhibited.[43] The TRPL spectra also show that the average emission lifetime of Cu4/Cu1@CN (1.85 ns) is longer than those of Cu1@CN (0.81 ns) and CN (0.85 ns). The increased PL lifetime of Cu4/Cu1@CN indicates a longer retention time of charge carriers. The excellent charge separation ability provides ample electrons for photocatalytic CO2 reduction.
In situ XAFS was used to probe into the alterations in the valence state of Cu during photocatalytic process. As shown in Figure 4a, upon the introduction of CO₂, a remarkable increase in the valence state of Cu was observed. This phenomenon implies that adsorption of CO₂ onto Cu is accompanied by the transfer of electrons to *CO₂. Subsequently, once the photocatalytic reaction commenced, the valence state of Cu was further elevated, signifying that a greater number of electrons were transferred from Cu to the reactants during the reaction course. In other words, CO₂ was reduced under the catalysis of Cu.
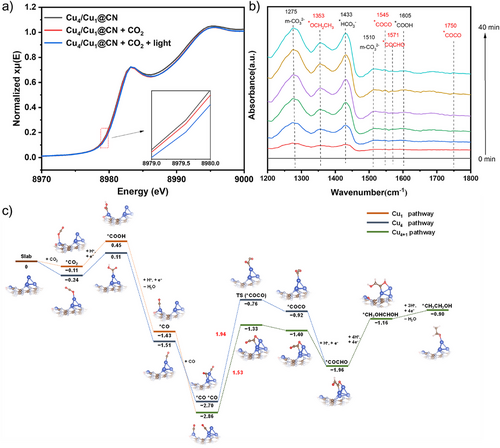
In situ attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS) was applied to monitor the possible intermediates in photocatalytic CO2 reduction. As shown in Figures 4b and S23, *OCH2CH3 (1353 cm−1),[44-46] *HCO3− (1433 cm−1),[47] m-CO32− (1275,1510 cm−1),[47, 48] *COCO (1545, 1750 cm−1), *COCHO (1571 cm−1),[18, 49] *COOH (1605 cm−1),[18, 49, 50] and *CO (2008, 2033, 2047 cm−1)[49] are observed. *COOH is the signature intermediate to form CO, *COCO is an essential intermediate for C2+ products, and *COCHO is a key intermediate to form ethanol. The presence of *OCH2CH3 is another important evidence that ethanol is produced in the reaction. The above results indicate that photocatalytic CO2 reduction to ethanol on Cu4/Cu1@CN occurs via *COCO to *COCHO and then to ethanol.
Wang and Li[22] have demonstrated through density functional theory (DFT) calculations that Cu4 clusters with three Cu─N2 bonds anchored on g-C3N4, which are consistent with the structure of the Cu4 clusters reported here, are capable of catalyzing the reduction of CO2 to C2+ products. In contrast, Cu SACs anchored on g-C3N4 are unable to drive this reaction due to the high energy barrier for CO─CO coupling. Their conclusion is in line with our experimental results, in which no ethanol or other C2+ products were observed with Cu1@CN as catalyst, whereas CH3CH2OH was produced with high rate in the system using Cu4/Cu1@CN.
We first calculated CO formation on both clusters and single-atom sites. As shown in Figure 4c, the free energy change (ΔG) for *COOH formation on both cluster sites (Cu4 pathway) and Cu single atom sites (Cu1 pathway) are very low, indicating that *CO is readily produced on both sites. To achieve C2+ products, C─C coupling is usually considered as the rate determine step. The C─C coupling on the Cu single atom site has been studied; however, *COCO was not stable on this site, indicating that Cu single atoms alone are not the active sites for C─C coupling. This is consistent with the experimental results that only CO and CH4 were obtained when Cu1@CN was used as the catalyst. Thus, two other pathways are considered for the C─C coupling on Cu4/Cu1@CN in our study: i) two *CO are both located on clusters (Cu4 pathway), and ii) two *CO coupling in a synchronized manner where one *CO is located on Cu4 cluster and the other one is on Cu single atom site (Cu4+1 pathway). For the Cu4 pathway, *CO is firstly absorbed on the vertex site of Cu4 cluster due to its more negative Gibbs free energy of *CO absorption (−1.51 eV, Figure S24), and the adsorption of a second CO molecule to form *CO−*CO is thermodynamically favored with ΔG = −1.19 eV. However, C─C coupling by the two adjacent *COs needs to overcome an energy barrier of 1.94 eV. For the Cu4+1 pathway, the energy barrier of C─C coupling between *CO located on Cu single atom site and the *CO on the nearby Cu4 cluster is 1.53 eV, lower than that in Cu4 pathway, indicating that the synergistic effect between Cu cluster and single atom promotes C─C coupling reaction. *COCHO can be readily produced by the hydrogenation of *COCO with ΔG = −0.56 eV. *COCHO is further reduced to *CHOHCH2OH with ΔG of 0.9 eV and then to the product *CH3CH2OH with an additional ΔG of 0.26 eV.
Effect of Cu Loading
To investigate the effect of Cu loading on photocatalytic performance, another two samples with mass ratios of Cu(NO₃)₂ to a-C₃N₄ of 15% and 60% were synthesized, and their photocatalytic performances were compared with the above reported 3% (Cu₁@CN) and 30% (Cu₄/Cu₁@CN) samples. As depicted in Figure S25, ethanol production demonstrated a volcano-type dependence on Cu(NO₃)₂ addition, peaking at 30% before declining. This trend is rationalized by a dual mechanism: incremental Cu loading could increase active site density, whereas excessive Cu deposition obscured the C₃N₄ matrix, compromising light absorption efficiency and photogenerated carrier separation. These observations are corroborated by complementary spectroscopic and electrochemical characterizations, including UV–vis DRS, transient photocurrent response, PL, and TRPL spectroscopy (Figures S26 and S27).
Conclusion
Reported Cu-based materials for photocatalytic CO2 to C2+ are mainly based on Cu single atoms or Cu-based alloys. In this work, we have designed a catalyst composed of Cu4 clusters together with adjacent Cu single atoms anchored on carbon nitride (Cu4/Cu1@CN). This material is a highly efficient CO2 to ethanol photocatalyst with production rate up to 154 µmol h−1 g−1 and selectivity of 98%, which are better than most reported photocatalysts for this conversion. We showed by experimental and theoretical calculations that the high efficiency of this catalyst arises from the strong synergy between Cu4 clusters and adjacent Cu single atoms. *CO can be formed efficiently on both single Cu and Cu4 sites. The C─C coupling energy barrier between *CO on a single atom and adjacent *CO on a Cu4 cluster is found to be much lower than that between two *CO on a Cu4 cluster.
Our work should provide valuable insights on the design of single Cu atoms and Cux clusters for the photocatalytic conversion of CO2 to C2+.
Supporting Information
Experimental and calculation procedures. Spectra and data. The authors have cited additional references within the Supporting Information.[51-59]
Acknowledgements
This research was funded by the National Natural Science Foundation of China (22102044, 22001253) and the Fundamental Research Funds for the Central Universities of China (PA2021KCPY0057).
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




