Oligo-Adenine Derived Secondary Nucleic Acid Frameworks: From Structural Characteristics to Applications
Abstract
Oligo-adenine (polyA) is primarily known for its critical role in mRNA stability, translational status, and gene regulation. Beyond its biological functions, extensive research has unveiled the diverse applications of polyA. In response to environmental stimuli, single polyA strands undergo distinctive structural transitions into diverse secondary configurations, which are reversible upon the introduction of appropriate counter-triggers. In this review, we systematically summarize recent advances of noncanonical structures derived from polyA, including A-motif duplex, A-cyanuric acid triplex, A-coralyne-A duplex, and T ⋅ A-T triplex. The structural characteristics and mechanisms underlying these conformations under specific external stimuli are addressed, followed by examples of their applications in stimuli-responsive DNA hydrogels, supramolecular fibre assembly, molecular electronics and switches, biosensing and bioengineering, payloads encapsulation and release, and others. A detailed comparison of these polyA-derived noncanonical structures is provided, highlighting their distinctive features. Furthermore, by integrating their stimuli-responsiveness and conformational characteristics, advanced material development, such as pH-cascaded DNA hydrogels and supramolecular fibres exhibiting dynamic structural transitions adapting environmental cues, are introduced. An outlook for future developments is also discussed. These polyA derived, stimuli-responsive, noncanonical structures enrich the arsenal of DNA “toolbox”, offering dynamic DNA frameworks for diverse future applications.
1 Introduction
Dictated by Watson–Crick base pairing,1 duplex helical DNA structures encode significant genetic information, transcribed into RNA and translated into proteins. Beyond the conventional A-T-G-C alphabet paired duplex DNA conformations (Figure 1a), external auxiliary stimuli prompt sequence-specific DNA strands to adopt secondary structures (Figure 1b, panel i, ii, iii). These include i-motif (a framework generated by cytosine-rich strands under mild acidic conditions),2 G-quadruplex (a quadruplex formed from guanine-rich strands in the presence of metal ions, such as K+, Sr+, or Pb2+),3 triplex (resulting from the protonation of auxiliary strands forming T ⋅ A-T or C+ ⋅ G-C structures),4 metal ions bridged duplexes (T-Hg2+-T, or C-Ag+-C),5 and trans/cis-azobenzene stabilized duplexes.6 These triggered/counter-triggered reconfiguration of oligonucleotides were broadly used in developing the area of DNA nanotechnology, materials science and engineering and summarized in excellent reviews.5, 7-9 Nonetheless, a systematic exploration of adenine-rich strands or oligo-adenine (polyA) derived secondary structures (Figure 1b, panel iv) and their applications remains lacking.
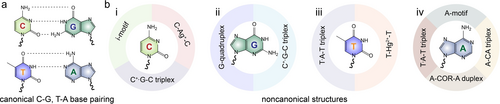
a) Schematic presentation of canonical C-G and T-A base pairing. b) Schematic demonstration of noncanonical structures derived from polyC (i), polyG (ii), polyT (iii), and polyA (iv) under external stimuli.
With an average length of approximately 200 nucleotides, polyA strands are synthesized by polyA polymerase (PAP) at the 3’-terminal of mRNA in eukaryotes. PolyA functions as a master regulator governing mRNA translational status and stability, along with cytoplasmic deadenylation and gene regulation, as reported in recent reviews.10-12 Beyond its pivotal biological roles,13 short polyA nucleic acids, composed of several tens of nucleotides, demonstrate diverse applications in materials science, biosensing/engineering, and nanotechnology. Due to its compositional and structural features, polyA can adopt distinct secondary configurations in response to various environmental stimuli, showcasing its multifunctional role in diverse research fields.
For example, under highly acidic conditions (pH<3.0), single-stranded polyA coils tend to form a parallel duplex structure known as A-motif,14, 15 bridged by protonated adenines (pKa 3.5). Its dissociation into single polyA strands occurs at a system pH higher than its pKa value, where deprotonation of adenine nucleotides takes place. These protonation/deprotonation processes are pH-reversible, leading to dynamic structural transitions. Additionally, small crescent-shaped molecules, such as coralyne (COR), bind to polyA forming high affinity complexes (Ka>107 M−1),16 inducing a secondary A-COR-A duplex structure distinct from the A-motif conformation. Moreover, cyanuric acid (CA), a low-molecular-weight cofactor with three complementary thymine-like edges, guides the self-assembly of single polyA strands into a noncanonical configuration (A-CA triplex) upon the protonation of CA ligands.17 The A-CA triplex disassembles upon deprotonation of CA cofactors. By employing polyT as the triplex-forming oligonucleotide, T ⋅ A-T triplex forms upon the protonation of auxiliary polyT strand (pH 7.0), undergoing separation upon deprotonation (pH 10.0).18 Moreover, competitive adsorption studies revealed superior affinity binding of polyA to Au surfaces, as compared to polyT, polyG and polyC strands.19 This specific polyA-Au interactions effectively compete against thiol-Au chemisorption, offering an alternative strategy for highly spatially controlled nucleic acid immobilization. Governed by polyA-Au adsorption, extensive studies have showcased its applications in precise engineering of nano-assemblies,20 biosensing,21 and catalysis.22 As polyA-Au interactions do not involve secondary DNA structures, the assembly of AuNPs by polyA strands will not be addressed in this review, yet reference to other relevant studies addressing this topic will be made.23
The present review provides an overview of the cutting-edge advancements in polyA-derived secondary nucleic acid structures and their extensive applications across various research domains. We discuss the structural characteristics and configurational transitions between single-stranded polyA and its secondary structures (e.g., A-motif, A-CA triplex, A-COR-A duplex, and T ⋅ A-T triplex) upon subjecting to external triggers/counter-triggers. We introduce applications that leverage these trigger-reversible dynamic structural transitions, including advanced triggered-cascaded configurational conversions, in materials science and engineering. Specific examples include stimuli-responsive DNA hydrogels, single molecular electronics, aptamer-ligand-like biosensing, supramolecular micro-fibres, payload encapsulation and delivery, constitutional dynamic networks (CDN), and more. Additionally, we offer insights into the challenges and opportunities associated with employing polyA in these research fields, aiming to identify further advancements beyond current boundaries and foster ongoing exploration of novel functional nucleic acid secondary structures for material science and bioengineering applications.
2 A-Motif Duplex
2.1 Structural Characteristics and pH-Responsiveness
The A-motif duplex, a nucleic acid secondary structure derived from polyA strands, forms under highly acidic conditions at pH lower than the pKa value of adenine (3.5).14 Under this condition, N1 protonation of adenine nucleotides promotes the assembly of two polyA strands into a parallel A-motif duplex (Figure 2a). This homogeneous right-handed A-motif is stabilized by protonated AH+−H+A units via both reverse Hoogsteen bonding and electrostatic interaction between the positively charged adenines and negatively charged phosphate groups of the backbone. The circular dichroism (CD) spectrum of A-motif exhibits an intense, positive band peaking at 264 nm with a shoulder at 272 nm and a negative band at 243 nm (Figure 2b). Upon raising the pH value above 3.5, deprotonation of adenines triggers the disassembly of A-motif into single polyA strands, stabilized by π–π stacking interactions of adenines. Consequently, the CD spectrum of polyA features a strong positive band peaks at 217 nm with a shoulder at 230 nm, a weak positive band observed at 270 nm, and negative bands centred at 250 nm and 205 nm (Figure 2b). Importantly, the pH-induced structural transitions between single polyA and A-motif duplex are reversible and reflected by alternating CD signals at 262 nm upon cycling the system pH between 3.0 and 7.0 (Figure 2c).
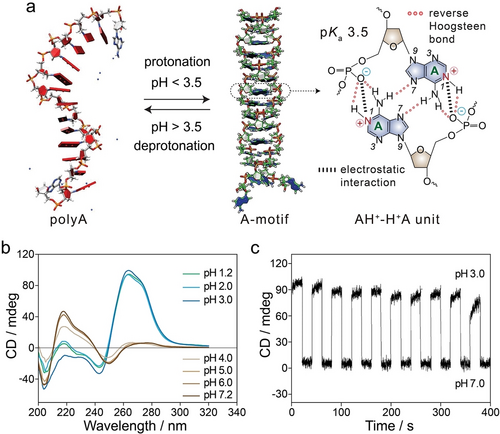
a) Schematic representation of pH-induced protonation/deprotonation of adenines resulting in A-motif duplex and single polyA strands. Reproduced with permission.14 Copyright 2009, Oxford University Press. b) CD spectra of polyA at pH values ranging from 1.2 to 7.2. Reproduced with permission.24 Copyright 2022, American Chemical Society. c) CD spectra recorded at 262 nm demonstrating the pH-reversible transitions between A-motif and polyA. Reproduced with permission.14 Copyright 2009, Oxford University Press.
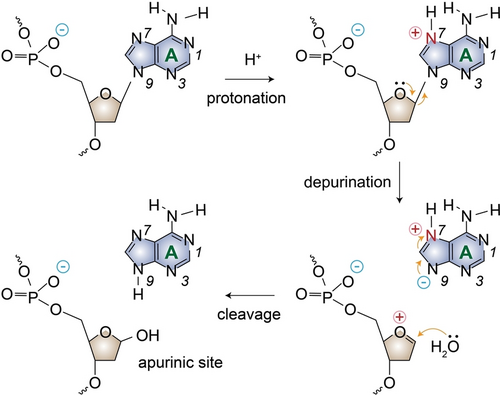
Schematic presentation of the depurination reaction and apurinic site generation leading to DNA cleavage under acidic conditions.
where k is in s−1 and T is the absolute temperature.
At pH 1.6, the half-life of polyA30 (t1/2=97 h) was 16.8-fold longer than that of polyAT15. In polyA strands containing repetitive adenine nucleotides, adenines are protonated at N1 position, acquiring positive charges under highly acidic conditions (e.g., pH<3.5). The high density of positive charges and the subsequent strands association into parallel A-motif duplex result in a slower depurination rate. Conversely, in polyAT15 strands, the minimal positive charge density has a negligible effect on the protonation of neighboring purines, leading to higher depurination rates. Gel electrophoresis results have, also, demonstrated the high stability of ployA strands when heated to 95 °C at pH 1.2.30 When melting at pH 1.2, the denaturing profiles of A-motif exhibited a well-defined sigmoidal thermal transition with Tm≈80 °C,14, 30 which is higher than that of A-T hybridized duplexes with the same sequence lengths (Tm≈60 °C).
2.2 A-Motif Based DNA Hydrogels
Based on its polymeric nature consisting of phosphate backbone and A-T-G-C alphabet, DNA has been widely used to construct functional DNA hydrogels/nanogels, in which the crosslinking of 3D polymer networks is achieved either through direct base-pairing or via specific secondary structures.31-33 Diverse applications in biomedical engineering, biosensing, gene editing, and shape modulation have been demonstrated.34-39 Recent advancements have explored the use of polyA strands to expand the capabilities of stimuli-responsive DNA hydrogels.
A recent study introduced a novel approach to creating a pH-responsive DNA hydrogel, utilizing A-motif duplexes as bridging elements.30 The process involved the synthesis of a copolymer, termed A-polymer, composed of a polyacrylamide backbone modified with polyA tethers. At pH 4.3, A-polymers existed in a liquid state, while adjusting the pH to 1.2 induced hydrogel formation through inter-bridging the polymer backbone by A-motif units. By recycling pH values between 4.3 and 1.2, reversible transitions between liquid and hydrogel states were demonstrated. Rheology studies confirmed the high thermal stability of the A-motif bridged DNA hydrogel, which reverted to a liquid state at around 100 °C.30 Moreover, the pH-responsive nature of this DNA hydrogel was exploited for controlled drug release, with the anti-inflammatory medication sulfasalazine intercalated into the A-motif duplex crosslinked hydrogel. Controlled release was achieved by adjusting the pH to 4.3, demonstrating the potential of this system for pH-controlled drug delivery applications.
An acid-resistant and physiological pH-responsive DNA hydrogel was further developed toward oral insulin delivery, utilizing A-motif and i-motif as the crosslinking elements.24 In this system, the DNA hydrogel was bridged by A-motif duplex at pH 1.2 and i-motif quadruplex at pH 5.2, allowing pH-triggered protonation of adenine and cytosine nucleotides, respectively. Adjusting pH value to 7.2 led to the deprotonation of both nucleotides and dissociation of crosslinking units, resulting in a liquid state and the release of insulin (Figure 4a). Scanning electron microscopy (SEM) images probing the 3D hydrogel networks under acidic pH conditions are displayed in Figure 4b. Rheology measurements confirmed the hydrogel states bridged by A-motif at pH 1.2 (storage modulus, G’=360±5 Pa, loss modulus, G”=2 Pa) and by i-motif at pH 5.0 (G’=96±10 Pa, G”=3 Pa) (Figure 4c). Two control hydrogels composed of solely polyA or polyC were also prepared to demonstrate the feasibility of the acid-resistant and physiological pH-responsive DNA hydrogel for oral insulin delivery, which passes through stomach (pH 1.2), duodenum (pH 5.0) and is released in the small intestine (pH 7.2). As shown in Figure 4d, the developed DNA hydrogel maintained its hydrogel state under acidic pH conditions with minimal dissociation (curve i), while fast and complete hydrogel disassembly was observed under physiological conditions (pH 7.2). In contrast, the A-motif crosslinked hydrogel consisting of solely ployA strands retained a hydrogel state at pH 1.2, which dissociated into solution at pH 5.0 (curve ii). Similarly, the i-motif bridged hydrogel composed of solely polyC strands dissociated immediately into a liquid state at pH 1.2 (curve iii). Subsequently, the in vivo pharmacokinetics and hypoglycemic effect of the insulin@DNA hydrogel were evaluated by oral administration to streptozotocin-induced diabetic rats. A significant decrease in blood glucose level was observed, and in vivo toxicity studies revealed excellent biocompatibility of the developed DNA hydrogel, which demonstrated its potential in drug delivery efficacies.
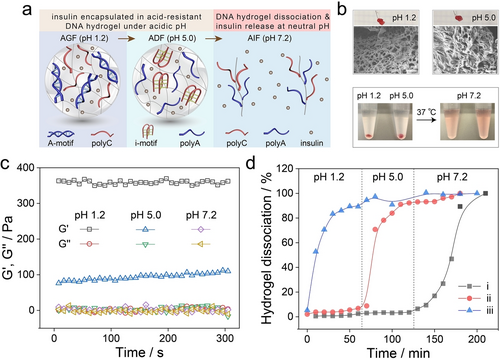
a) Schematic presentation of the DNA hydrogel crosslinked by A-motif duplex at pH 1.2, i-motif quadruplex at pH 5.0, and its dissociation into a liquid state at pH 7.2 accompanied by the release of encapsulated insulin. b) Photos and SEM images of the hydrogel states at pH 1.2 and pH 5.0, as well as its dissociation into solution at pH 7.2. c) Rheology measurements of the DNA hydrogel at pH 1.2, pH 5.0, and pH 7.2. d) The dissociation profiles of DNA hydrogels composed of both polyA and polyC (curve i), solely polyA (curve ii) or polyC (curve iii). Reproduced with permission.24 Copyright 2022, American Chemical Society.
2.3 A-motif Facilitated DNA Conjugation to AuNPs
Anchoring thiol-modified DNA strands to AuNPs via Au-S covalent binding is a widely employed strategy in various applications, including colorimetric sensing/biosensing, nano-assemblies, delivery vehicles, and biomedical therapies.40-42 Size-controlled AuNPs are typically synthesized using a citrate reduction method and stabilized by the negatively charged citrate ions adsorbed on the surface.43 Nonetheless, electrostatic repulsions between both negatively charged AuNPs and DNA strands perturb the exchange of the citrate layer by the thiolated DNA strands. To overcome this limitation, stepwise addition of salts, reducing the electrostatic reaction, was introduced,44, 45 yet this method is accompanied by destabilization and precipitation of AuNPs.
Within the efforts to modify AuNPs with oligonucleotides, binding of thiolated parallel A-motif duplex at low pH value (3.0) to AuNPs demonstrated high efficiency (Figure 5).46, 47 Adjusting the pH of the A-motif duplex-functionalized AuNPs to 7.0 separated the duplexes, allowing the modification of AuNPs by any tandem oligonucleotide sequence through a polyA spacer.
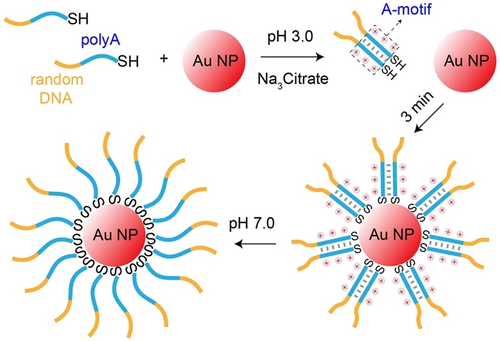
A-motif facilitated anchoring of thiolated DNA to AuNPs. Reproduced with permission.46 Copyright 2016, American Chemical Society.
2.4 A-Motif Facilitated DNA Probes for Biosensing
The structural transitions between single polyA strands and A-motif duplexes were applied as a probing mechanism to develop a pH-variation sensor (Figure 6a).48 A polyA strand comprising 20 consecutive adenines was modified with a fluorophore/quencher (Cy3/BHQ-2) pair at the 3’ and 5’ termini. Under neutral conditions (pH 7.0), polyA strands existed in a random coil conformation, where the spatial distance inhibited the efficient fluorescence resonance energy transfer (FRET) from Cy3 to BHQ-2, resulting in high fluorescence. However, under acidic conditions (pH 3.0) and a low polyA concentration (20 nM), protonation of adenines generated AH+−H+A base-pairing units, resulting in a folded intramolecular-stabilized A-motif duplex. The proximity between Cy3 and BHQ-2 facilitated efficient FRET and fluorescence quenching.
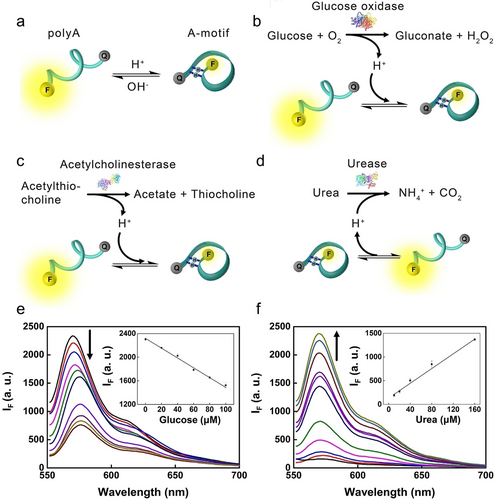
a) Schematic presentation of pH-induced structural transitions between polyA random coil and self-folding A-motif duplex. Enzymes mediated catalysis of substrates altering pH values, including b) GOx, c) AChE, and d) urease. Fluorescence detection of e) glucose and f) urea. Reproduced with permission.48 Copyright 2018, Elsevier.
The self-folding A-motif based DNA probe was applied to sense pH variations associated with biocatalytic interactions, including glucose oxidase (GOx) catalysing glucose in the presence of O2 into gluconic acid and H2O2, the hydrolysis of acetylthiocholine by acetylcholinesterase (AChE) into thiocholine and acetate, and the hydrolysis of urea by urease into NH4+ and CO2. The former two reactions generate acid, lowering the system pH values, which facilitate the formation of intramolecular A-motif duplex and fluorescence-quenching of Cy3 (Figure 6b and 6c). On the contrary, urease mediated hydrolysis of urea generates NH4+, which elevated the system pH value, leading to the separation of intramolecular A-motif into single polyA, restoring the fluorescence of Cy3 (Figure 6d). Utilizing these mechanisms, glucose within a linear concentration range of 20 to 100 μM was demonstrated with a limit of detection (LOD) of 6 μM (Figure 6e), comparable to other DNA sensors involving i-motif,49 molecular beacon,50 and G-quadruplex.51 Similarly, urea was detected within a linear concentration range of 10 to 160 μM with a LOD of 3 μM (Figure 6f). Moreover, the A-motif based DNA probes were applied to develop enzymes-mediated NOR and NAND logic gates.
By anchoring 5’ terminal thiol-modified polyA strands onto AuNPs (13 nm), a colorimetric pH sensor was developed.52 The aggregation and disaggregation of AuNPs were finely controlled by pH-induced formation and dissociation of A-motif duplex crosslinkers. Moreover, the pH-responsiveness of the colorimetric sensor could be tuned by incorporating mismatches into the polyA strands.
In contrast to other DNA-based sensors, which usually rely on added salts for proper functioning, the A-motif-based sensing platform distinguishes itself as the sole system capable of operating effectively in salt-free or extremely low salt conditions. This feature holds particular significance for pH sensing in lower ranges, which is pertinent in scenarios like environmental pollution or industrial toxification.52
2.5 A-Motif Mediated DNA Architectures
In constructing DNA architectures, Watson–Crick base-paring interactions are typically employed to build scaffolds, such as tetrahedra,53, 54 and polyhedra.55 When incorporating DNA functional motifs, such as i-motif,56, 57 G-quadruplex,58 aptamer-ligand,59 into these scaffolds, externally modulatable and dynamic DNA architectures are achieved. Similarly, by utilizing A-motif as dynamic crosslinking elements, the assembly and disassembly of 1D DNA architectures have been demonstrated, Figure 7.60 A DNA three-way junction with polyA overhangs was initially designed. At pH 3.5, protonation of the polyA overhangs led to the formation of A-motif duplex crosslinkers, resulting in the generation of a rigid DNA architecture. Under neutral conditions (pH 7.5), separation of A-motif “sticky ends” caused the disassembly of the architecture into its constituent building blocks. The reversible pH-driven reconfiguration was demonstrated. Moreover, by incorporating thiol groups into the building blocks, the DNA architecture mediated assembly/disassembly of AuNPs was also demonstrated. This study illustrates the methodology of incorporating simple and non-interfering polyA strands into non-B DNA structures, achieving their controlled assembly. This approach could be further extended to the fabrication of functional 2D or 3D DNA architectures.
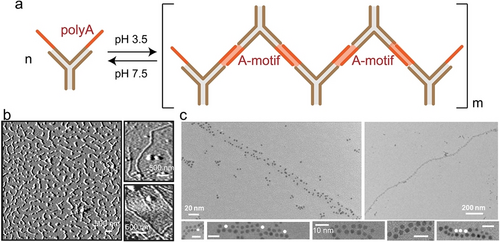
a) Schematic presentation of A-motif mediated reversible assembly/disassembly of DNA three-way junctions. b) Atomic force microscopy (AFM) images of the assembled 1D DNA architectures. c) Transmission electron microscopy (TEM) images of AuNPs assemblies mediated by the DNA architectures. Reproduced with permission.60 Copyright 2010, Wiley-VCH GmbH.
3 Cyanuric Acid (CA) Mediated A-CA Triplex
3.1 Structural Characteristics and pH-Responsiveness
Low-molecular-weight cofactors, such as cyanuric acid (CA, pKa 6.9), a symmetric and nontoxic ligand with three complementary thymine-like edges, have the ability to reprogram the self-assembly of single polyA strands into a novel noncanonical DNA architecture termed A-CA triplex. This structure is held together by a hydrogen-bond network between adenine nucleotides and protonated CA molecules (Figure 8a).17, 61-65 The formed parallel A-CA triplex adopts a helicene-like configuration within a pH range of 4.0–7.0, where adenine (pKa 3.5) is uncharged, and CA molecules are protonated. At pH 5.2, a noticeable hypochromic effect is observed in the absorption band of polyA at 260 nm, accompanied by a slight red-shift, indicating increased base-stacking interactions upon the formation of CA-mediated polyA assembly (Figure 8b).66 Additionally, the rising of absorbance at λ=285 nm is indicative of the generation of J-aggregates due to the stacking of ordered chromophore.17, 67 The CD spectrum of A-CA triplex exhibits a strong negative band peaking at 250 nm and a weak positive band at 268 nm (Figure 8c, pH 5.2), demonstrating a triplex configuration by A-CA helicene architectures via hydrogen bonds involving both Watson–Crick and Hoogsteen interactions, similar to T ⋅ A-T configuration.4 The stoichiometry of CA to adenine was determined to be 1 via equilibrium dialysis studies, consistent with a 1 : 1 CA : adenine ratio in the helicene-like configuration.17
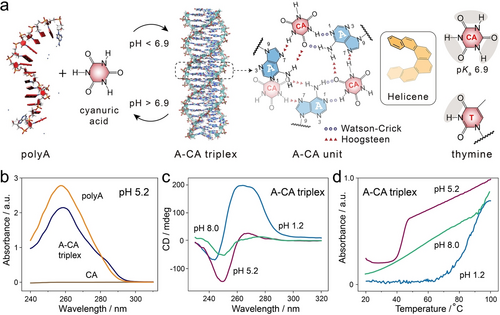
a) Schematic presentation of CA-mediated formation of parallel A-CA triplex under mild acidic conditions (pH<6.9). The chemical structure of an A-CA helicene unit linked by hydrogen bonds and the molecular structure of CA with thymine-like edges are shown on the right. The A-CA triplex structure was reproduced with permission.63 Copyright 2020 Wiley-VCH GmbH. b) UV-Vis spectra of single polyA, CA and A-CA triplex at pH 5.2. c) CD spectra and d) melting profiles of A-CA triplex under conditions of pH 1.2, 5.2, and 8.0. Reproduced with permission.66 Copyright 2024, American Chemical Society.
The denaturation profiles of A-CA triplex exhibit a clearly defined cooperative transition with a sigmoidal pattern (Figure 8d, pH 5.2), indicating the thermal disassembly of CA-mediated A-CA triplex into its constituents. The sharp transition occurring within less than 10 °C (from 40 °C to 48 °C) demonstrates the direct dissociation of homogeneous A-CA triplex configurations without any intermediate stages. Dissociation of A-CA triplex occurs under physiological conditions (pH 8.0) due to the deprotonation of CA cofactors. The 280 nm band in CD spectrum originates from a forbidden (n–π*) transition, indicating that single polyA strands are predominantly stabilized by π–π stacking of adenines, which is verified by the broad and noncooperative melting profiles of A-CA triplex at pH 8.0. It should also be noted that, at pH 1.2, A-CA triplexes transition into A-motif duplex due to the protonation of adenines, as evidenced by the characteristics of CD spectrum (Figure 8c) and melting profiles (Figure 8d). The Tm of A-CA triplex (≈43 °C) was approximately half that of A-motif (Tm≈85 °C).
3.2 Supramolecular Micron-Sized Fibres
Since adopting DNA as functional material for nanotechnology,68 numerous studies have been conducted, showcasing the vast potential of DNA across diverse research fields and applications.23, 69-72 The A-T-G-C alphabet-dictated base-pairing offers the advantages such as programmability, enabling the constructions of complex architectures like DNA origami,73 nanorobots,74 or nano-assemblies.75
A-CA triplex based supramolecular micron-sized fibres were fabricated by employing CA molecules as staple units and polyA strands as the skeleton.17 At pH 4.5, protonation of CA cofactors mediated the assembly of polyA into supramolecular fibres via either blunt-ended stacking or staggered constructs, with fibre elongation further achieved by the polyA overhangs (Figure 9a and 9b). The supramolecular fibre assemblies were found to be influenced by both CA concentration and the strand length of polyA. Within the polyA concentration range of 25–75 μM, a critical concentration of CA was obtained at 3 mM (CA : adenine ratio >3 : 1), below which no fibre assembly occurred (Figure 9c). These results confirmed that the polymerization mechanism involves cooperative nucleation-growth. The elongation of fibre nuclei, likely small structures with staggered strands, into higher-order structure occurs only above the critical concentration of CA molecules (inset of Figure 9c).
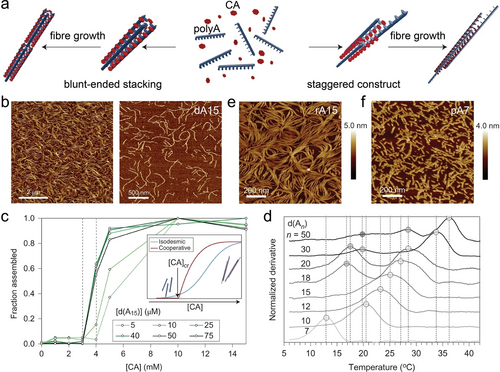
a) Schematic presentation of supramolecular A-CA triplex fibre elongation modes, including blunt-ended constructs and staggered constructs. b) AFM images of supramolecular DNA (dA15) fibres. c) Fraction of dA15 participating in the assembly as a function of CA concentrations. d) Melting temperatures of CA-mediated supramolecular assemblies from dAn (n from 7 to 50) with CA concentration of 10 mM. AFM images of supramolecular A-CA fibres derived from e) RNA (rA15) and f) PNA (pA7) in solution. Reproduced with permission.17 Copyright 2016, Springer Nature Limited.
The kinetics and thermodynamic properties of the A-CA triplex assemblies were also affected by the length of polyA strands. For polyA with nucleotides less than 15 (n≤15), the A-CA structures exhibited monophasic melting transitions. As the polyA length increased (n≥18), multiphase melting transitions were observed (Figure 9d). When short polyA strands assemble in the presence of CA cofactors, they tend to shift and associate uniformly during polymerization, resulting in a homogeneous assembly of supramolecular structures with approximately same melting temperature. Conversely, for longer polyA strands, there are multiple possibilities for the long chains to overlap between polyA strands, allowing for the elongated formation of fibres, resulting in a distribution of fibre exhibiting multistep melting transitions. Generally, the kinetics of supramolecular assemblies was enhanced at higher concentrations of CA and longer polyA strands. Moreover, besides DNA, polyA composed of RNA (rA15) or PNA (pA7) were, also, assembled into supramolecular fibres, in the presence of CA cofactors (Figure 9e and 9 f), demonstrating the versality of CA-induced assembly of nucleic acid structures.
In the A-CA triplex assembly, two of the three thymine-like edges of CA cofactors are involved in forming the helicene-like configuration, offering the possibility of introducing functional side chains to CA. Substituted CA (CA-R) molecules with OH or NH2 terminals of varying side chain lengths (Figure 10a) were systematically investigated for their interactions with polyA into triplex configurations.61 It was demonstrated that the CA-Rs successfully assembled polyA into triplex structures (Figure 10b), subsequently forming supramolecular micron-sized fibres with alkylamine or alcohol units. Significant deformations in polyA sugar and phosphate backbones were observed facilitating hydrogen bond formation between the phosphate groups and CA-R side functionalities (Figure 10c). Additionally, a critical chain length of the side functionalities transforming the interaction from intra- to inter-fibre was demonstrated. Particularly, CA-Rs with positively charged amino functionalities exhibited stronger binding with the negatively charged phosphate backbones of polyA strands compared to CA-Rs with neutral alcohols (Figure 10d). The free energy trend for amines showed a non-monotonic behavior with chain length, where CA-C2NH2 and CA-C5NH2 were better binders than CA-C3NH2. Overall, the interactions between polyA phosphate groups and amines of CA-R side chains introduced conformational changes in the assembled A-CA triplex, which are particularly intriguing for adjusting both the mechanical and, ultimately, the biological properties of the CA-mediated DNA supramolecular polymers.
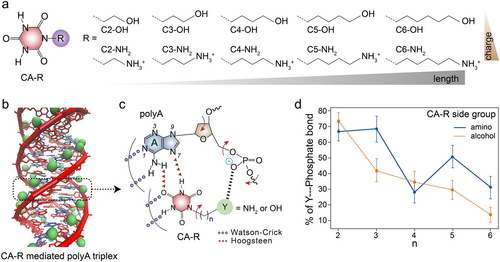
a) Chemical structures of sidechain substituted CA (CA-R). b) Schematic presentation of CA-R mediated assembly of polyA into triplex structures. c) Schematic interactions between side functional groups of CA-R and phosphate groups of polyA. d) Percentage of polyA phosphate groups forming a hydrogen bond with the CA-R of amino and alcohol side groups. Reproduced with permission.61 Copyright 2021, American Chemical Society.
Supramolecular A-CA triplex fibres demonstrated high resistance towards nuclease degradation,76 offering a path to protect DNA and RNA using small molecule mediated structures. Mechanisms proposed to interpret the enzyme-resistance include the inability of enzymes to recognize A-CA triplex structures and the inaccessibility of sequences incorporated in these supramolecular fibres to nucleases. However, these experiments were conducted under acidic conditions (pH 5.7–6.2), where the protonation of CA cofactors is essential to assemble polyA strands into A-CA triplex structures. A reduced protection effect of sequences was observed at pH 7.2 due to the deprotonation of CA inducing disassembly of supramolecular structures.
3.3 Stimuli Responsive Hydrogel
In addition to the A-CA triplexes based supramolecular polymers and fibres,17, 61, 65 DNA hydrogels revealing high stiffness were prepared.77 Typically, DNA hydrogels consisting of unmodified DNA or pure DNA strands are very soft, with G’ values in the range of 101 ~103 Pa, limiting their applications in soft robotics or extracellular matrix uses. Utilizing noncanonical A-CA triplexes was suggested to resolve these limitations.77, 78
As depicted in Figure 11a, an unmodified DNA basic construction unit, comprising a hybridized duplex domain and two polyA overhangs, was designed. At pH 6.0 and in the presence of CA molecules, CA-mediated self-assembly of 3D crosslinked porous polymer networks forming DNA hydrogel was demonstrated (Figure 11b). The resulting DNA hydrogels exhibited a high stiffness (G’ of 105 Pa), yielding the stiffest unmodified DNA hydrogels revealing two orders of enhanced magnitude as compared to other DNA hydrogels. This exceptional stiffness may be attributed to the high density of integrated CA cofactors along DNA backbone. The supramolecular A-CA structure facilitated fast and efficient self-healing of the DNA hydrogel (Figure 11c). Additionally, this A-CA triplex based hydrogel exhibited multi-stimuli-responsiveness to polyT sequences (forming A-T duplex), basic pH (CA deprotonation) and small molecules, such as melamine (MA, forming hexameric rosette architectures with CA via hydrogen bonds), with a state transition from hydrogel to liquid (Figure 11d). Moreover, the CA-mediated DNA hydrogels were engineered into delivery vehicles for anti-sense oligonucleotides (ASO), capable of silencing the reporter luciferase gene in a HeLa cell line (Figure 11e). The release of ASO from hydrogels was triggered by physiologically relevant pH in the cellular environment. After binding to its target mRNA, ASO inhibited its translation into luciferase, leading to a low luminescence signal (Figure 11f). Following a 48-hour incubation period, the silencing efficiency of ASO varied between 84±1 % and 93±1 % depending on the method of incorporation, with the free strand physically encapsulated in the DNA hydrogel exhibiting the highest activity, and the complementary strand showing the lowest activity, due to the competition in binding between the complement to the ASO and the mRNA (Figure 11g).
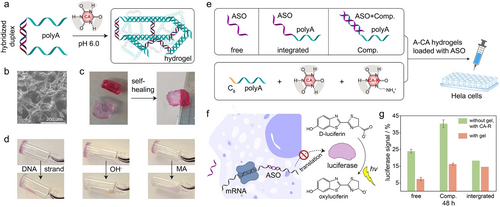
a) Schematic presentation of generating DNA hydrogel from CA-mediated assembly of unmodified duplex DNA with polyA overhangs at pH 6.0. b) SEM image of the DNA hydrogel. c) Self-healing properties of the hydrogel. d) Multi-stimuli-responsiveness of the A-CA triplex DNA hydrogel triggered by polyT strands, basic pH, and small molecules (MA). e) A-CA triplex DNA hydrogels loaded with ASO by direct physical encapsulation (free), integration to polyA (integrated) and hybridization to its complementary counterpart of polyA overhang (Comp.). f) Schematic demonstration of ASO binding to mRNA and inhibiting its translation into luciferase, which catalyses D-luciferin to oxyluciferin with the generation of luminescence. g) Comparison of the normalized luciferase signal from different ASO incorporation methods into the DNA hydrogels as illustrated in e). Reproduced with permission.77 Copyright 2023, Wiley-VCH GmbH.
Additionally, a Y-shaped DNA building block with three polyA overhangs and one short single polyA strand were assembled into a hydrogel mediated by CA cofactors.79 The A-CA bridged hydrogel served as a carrier for various payloads, including TAMRA (N,N’-tetramethylrhodamine), doxorubicin (DOX), AuNPs, iron oxide nanoparticles (MNP-cy5.5 or MNP-DOX), and streptavidin, as confirmed by fluorescence or digital imaging. Moreover, two different model components (red-avidin and green-avidin) were encapsulated at different layers of the CA-crosslinked hydrogel, demonstrating the capability of sequential payloads encapsulation.
4 A-COR-A Duplex
4.1 Structural Characteristics
In addition to thymine-edge-like cofactors, other protoberberine-type alkaloids, such as coralyne (COR), also induce the assembly of polyA strands into noncanonical DNA structures under neutral pH conditions.16, 80 COR molecules reveal superior antileukemic abilities and are utilized as anticancer drug with low toxicity.81 Furthermore, based on its structural characteristics, COR intercalates into DNA/RNA triplexes with a preference to T ⋅ A-T or U ⋅ A-U triplets, respectively.82-84 It has been observed that, in the presence of COR and under elevated temperature, the A-T duplex undergoes irreversible transaction and disproportionation into T ⋅ A-T triplex and COR induced anti-parallel polyA secondary structure (A-COR-A duplex).83, 85 Molecular dynamics simulations and coupled nucleotide substitution experiments have verified the characteristic features of the duplex structure, which involves N7 in adenine and adopts a Watson–Crick Hoogsteen (transWH) geometry (Figure 12a), considering energetics, structural stability, and hydrogen bonding.86 The stable A-COR-A anti-parallel duplex exhibits a Tm value of 60 °C determined by CD measurements.16
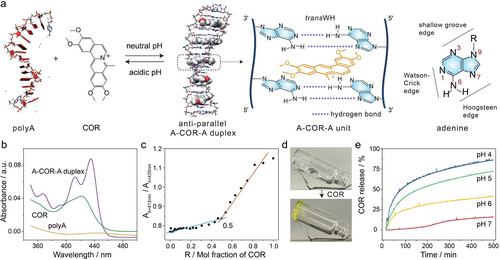
a) Schematic presentation of COR-induced assembly of polyA strands into anti-parallel A-COR-A duplex under neutral pH conditions and its acidic pH triggered disassembly. The A-COR-A duplex configuration was reproduced with permission.86 Copyright 2009, Oxford University Press. The transWH geometry of the A-COR-A unit is presented on the right. b) UV-Vis spectra of polyA, COR and A-COR-A duplex. c) Job plot of COR with polyA, in which an inflection point of 0.5 is observed, indicating a binding level of one COR molecule per four adenine bases. R (horizontal axis) represents the molar fraction of COR, R=[COR]/([COR]+[polyA in adenine bases/4]). Aλ=412nm/Aλ=435nm (vertical axis) is the absorption ratio of COR recorded at 412 nm versus 435 nm. d) Photos of COR induced hydrogel state. e) Time-dependent release profiles of COR from the A-COR-A duplex bridged DNA hydrogel triggered by pH variations. Reproduced with permission.89 Copyright 2024, American Chemical Society.
Characterized by UV-Vis spectroscopy, the A-COR-A duplex exhibits distinct absorbance characteristics peaking at 367, 412, and 435 nm differing from separated COR or polyA (Figure 12b). Additionally, the formation of A-COR-A was confirmed by the CD spectrum with the heightened intensity of the band at 278 nm and the appearance of a positive band at 335 nm.87 Furthermore, the stoichiometry of the complex was determined through continuous fraction analysis (Job plot) by plotting the ratio of COR absorbance at 412 nm to 435 nm against the relative concentration of COR to A-strand (in adenine bases/4), while maintaining the total concentration constant at 50 μM (Figure 12c).83, 88 An inflection point of 0.5 was obtained, indicating a conjugation level of one COR molecule per four adenine bases in the configuration of A-COR-A duplex (Figure 12a). The stability of A-COR-A duplex decreases with decreasing system pH values (from 7.0, 6.0, 5.0, 4.0 to 3.0), as indicated by the reduction of the positive CD band at 335 nm.87
4.2 pH-Responsive A-COR-A Based Delivery Platforms
The structural transitions between single-stranded polyA and the A-COR-A duplex introduced a novel pH-responsive DNA hydrogel mediated by COR.89 In the absence of COR, the copolymer with a polyacrylamide backbone and polyA branches existed in a liquid state due to the absence of crosslinking elements (Figure 12d). However, introducing COR molecules led to a hydrogel state crosslinked by A-COR-A duplex (Figure 12d). The DNA hydrogel exhibited acidic pH-triggered responsiveness, as evidenced by the rapid COR release rate under acidic conditions (Figure 12e).
Compared to other drug release systems based on pH-responsive DNA hydrogels, the A-COR-A duplex presents several advantages: i) It expands the repertoire of pH-responsive DNA hydrogels beyond the conventional A-T-G-C alphabet-based systems. Typically, pH-responsive DNA hydrogels are constructed using limited DNA configurations, such as i-motif, triplex (C+ ⋅ G-C or T ⋅ A-T), and A-motif. Identifying alternative stimuli-responsive DNA hydrogels is challenging, which was indeed addressed in a recent report on A-CA triplex based hydrogels.77 ii) The A-COR-A bridged DNA hydrogel provides a direct and well-defined drug cofactor-driven hydrogel state, allowing precise control over drug-loading at a defined stoichiometry of one COR cofactor per four adenines. This approach contrasts with methods relying on physically entrapped drugs within 3D hydrogel frameworks.90, 91 iii) Under acidic pH conditions, direct decomplexation of COR from A-COR-A units leads to drug (COR) release and hydrogel dissociation.
Moreover, the pH-triggered disassembly of A-COR-A duplex into single polyA strands, accompanied by the simultaneous release of COR molecules, has been applied to treat cancer cells by using gold nanorod or mesoporous silica NPs.92, 93 Through the formation of A-COR-A coordination, COR molecules were loaded onto polyA strands modified gold nanorods. Upon internalization of the NPs in the acidic compartments of cancer cells (lysosome, pH∼5.0), the dissociation of A-COR-A duplexes released COR for cancer chemotherapy. Meanwhile, the conversion of near-infrared (NIR) light by gold nanorods into heat provided additional hyperthermal cancer therapy and accelerated the triggered release of COR.92 Similarly, both COR and indocyanine green (a fluorescent dye capable of converting NIR light into heat) were loaded in the pores of silica NPs and capped by A-COR-A complexes as gates. Upon endocytosis into cancer cells, both acidic pH and NIR light facilitating the release of COR and indocyanine green for effective chemothermal cancer therapy was demonstrated.93
4.3 COR Mediated DNA Molecular Rectifiers and Switches
Due to its inherent structural characteristics, molecular recognition and programmability, DNA emerges as an ideal candidate for applications in molecular electronics and spintronics.94 Studies have investigated the electron transport through DNA, revealing both short-range coherent tunnelling95 and long-range incoherent hopping mechanisms.96 Moreover, an intermediate regime of tunnelling–hopping has been identified,97 facilitated by strong coupling of π-electrons between adjacent base pairs, leading to the delocalization of holes across multiple base pairs in DNA. In addition, research into coherent spin transport within DNA,98 involving configurational transition or oxidative damage,99, 100 underscores the significance of DNA structures as efficient spin filters in spintronics.
DNA intercalators, such as aromatic molecules, can intercalate in-between base pairs, leading to the perturbation of DNA duplex structures, thereby rearranging the electron density along the π–π-stacked base pairs, resulting in conductance alterations and rectification. For instance, by intercalating COR molecules into a specifically designed DNA duplex, a COR-mediated DNA supramolecular rectifier was developed.101 Electrical measurements of the single DNA-COR supramolecular junction by using scanning tunnelling microscopy break junction (STMBJ) technique are depicted in Figure 13a. The 3’ end thiol group facilitated junction attachment to Au (111) surface via specific Au−S bonding. UV-Vis, Job plot and CD studies indicated that two COR molecules intercalated between the mismatched adenines to form a stable, symmetric B-DNA supramolecular structure. Intercalation of the π-conjugated COR units into the DNA duplex perturbated the π–π-stacked adjacent base pairs, thereby modulating the conductance of the duplex framework. Within the bias voltage range of −1.1 to 1.1 V, the I–V curve of native DNA exhibited symmetrical current behavior under opposite bias polarities (Figure 13b). In contrast, the DNA-COR supramolecular structure revealed significant asymmetrical I–V behavior, showing a sharp current increase at negative bias, indicative of molecular rectification. The rectification behavior was highly counterintuitive given the symmetric supramolecular conformation of the DNA-COR complex. The supramolecular DNA-COR junction displayed small rectification ratio (RR) values slightly above unity under low bias (∼0–0.7 V). However, these values significantly increased when the bias exceeded 0.7 V (defined as the switch-on voltage), eventually jumping to ∼15 at 1.1 V (Figure 13c). This value surpassed the typical RR values (∼2–10) recorded for symmetric molecules-based junctions,102 and approached the theoretically estimated upper limit of RR (∼20) in a coherent transport molecular junction.103 This study introduced a new method for investigating DNA-small molecule interactions and proposed a novel strategy for engineering molecular electronics via noncanonical DNA-COR conformations, which could inspire exploration of other configurations, such as A-motif or A-CA triplex.
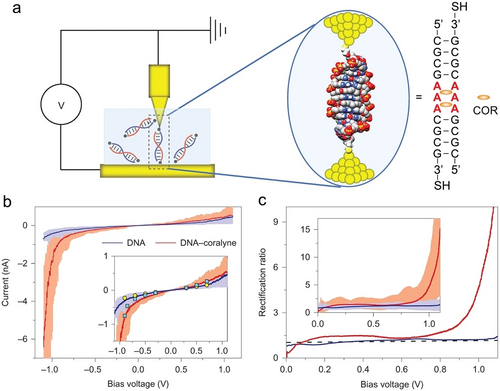
COR facilitated DNA molecular rectifier. a) Schematic presentation of the STMBJ system. b) Average I–V curves (solid line) alongside the 40 individual curves (light shadow) for both native DNA (blue) and DNA-COR complex (red). Inset: Graph showing an overlay of I–V curves with static current values represented by yellow circles for native DNA and cyan squares for the DNA-COR complex, under variable bias voltages. c) Comparison of average rectification ratios between native DNA (blue) and the DNA-COR complex (red). Inset: Comparison of average rectification ratios between native DNA and the DNA-COR complex across 40 individual rectifications versus bias voltage curves. Reproduced with permission.101 Copyright 2016, Springer Nature Limited.
Incorporating chromophores into nucleic acids unveils unique photophysical and structural properties far beyond those of the individual chromophores in their monomeric states. These properties include the formation of supramolecular excimers and exciplexes with distinct fluorescence and charge transfer properties, spatially controlled orientation and communication, and more.104-106 These features were applied for developing supramolecular assemblies, photonic, electronic, and molecular devices.107-109 Nevertheless, one of the changes rests in the controlled dynamic assembly/disassembly of chromophore-nucleic acids constructs beyond conventional methods.110, 111 To address this goal, an A-COR-A duplex was employed to assemble chromophore-guided DNA duplexes.112 The adenines of polyA strands were modified with a chromophore, pyrene (Py), which directed the formation of a self-duplex via the stacking of PyA units. With the introduction of COR into the system, disassembly of the duplex into single polyPyA strands was demonstrated, due to the competitive π–π stacking intercalation between Py and COR as a 1 to 1 host–guest binding complex (Figure 14a). Moreover, a DNA molecular switch stabilized by PyA units would selectively transition to an A-COR-A duplex structure through the intercalation of COR molecules between unmodified adenines (Figure 14b). Although reversibility was not achieved in these systems, this study introduced new avenues for controlling the conformations and photonic properties of chromophore-nucleic acids by introducing intercalators, thereby inspiring the development of switchable supramolecular photonic devices.
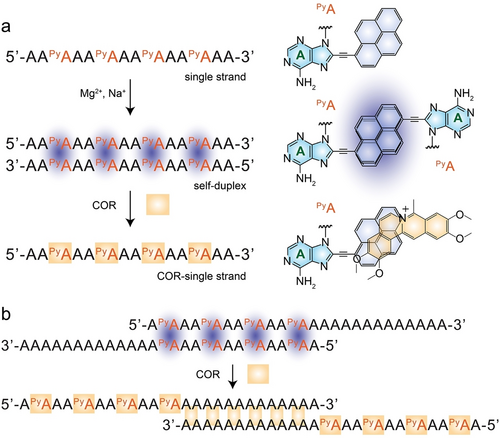
COR mediated DNA molecular switches. a) Schematic presentation of COR induced disassembly of polyPyA self-duplex into single COR-polyPyA strands. The structures of PyA, PyA dimer and COR-PyA are illustrated on the right. b) Schematic demonstration of COR induced structural transition of polyPyA self-duplex into A-COR-A duplex. Reproduced with permission.112 Copyright 2016, Wiley-VCH GmbH.
4.4 A-COR-A for Biosensing
Relaying on COR-induced structural transitions of single polyA strands to A-COR-A antiparallel duplex, various sensing platforms were realized, including colorimetry, fluorescence, electrochemistry, surface plasmon resonance (SPR), dual polarization interferometry (DPI), and surface enhanced Raman scattering (SERS) as transduction means.81 The high binding affinity exhibited by the A-COR-A duplex (Ka>107 M−1)16 renders polyA as suitable framework for the COR cofactors. For example, in the absence of COR, polyA strands adsorb onto silver nanoparticles (AgNPs) with a high affinity, preventing salt-induced aggregation of AgNPs.113 Conversely, in the presence of COR, polyA strands preferentially bind to COR to form A-COR-A duplexes, resulting in naked AgNPs that aggregate upon subsequent addition of salt, leading to a color change from yellow to brown (Figure 15a). This colorimetric detection method showed a good linear correlation with COR concentration within the range of 0 to 10 μM and demonstrated high selectivity towards COR against ethidium bromide and daunomycin. A similar strategy was developed for colorimetric detection of COR by using AuNPs.114 PolyA modified AuNPs aggregated in the presence of COR, resulting in a color change from red to blue.115 The introduction of active ricin caused the depurination of ployA strands into multiple adenines and inhibited the formation of A-COR-A duplexes, thereby preventing the aggregation of AuNPs (Figure 15b), which served as a platform for the detection of bioactive ricin. Additional colorimetric detection platforms for COR include citrate-capped AgNPs,116 and DNA functionalized AuNPs.117
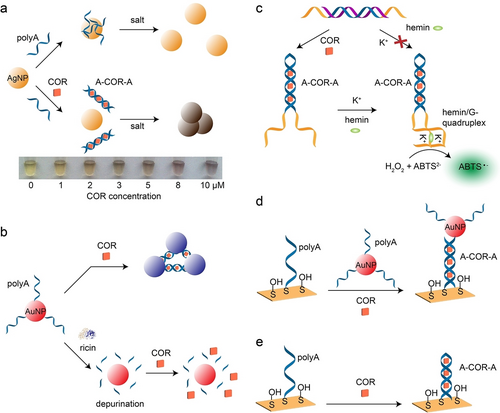
a) Colorimetric assay of COR using salt-induced aggregation/disaggregation of AgNPs. Reproduced with permission.113 Copyright 2009, Wiley-VCH GmbH. b) Colorimetric detection of active ricin by using polyA modified AuNPs. Reproduced with permission.115 Copyright 2017, American Chemical Society. c) Colorimetric assay of COR by hemin/G-quadruplex catalysed oxidization of ABTS2− to ABTS⋅− in the presence of H2O2. Reproduced with permission.118 Copyright 2013, Royal Society of Chemistry. d) SPR based detection of COR by polyA modified AuNPs. Reproduced with permission.121 Copyright 2012, Chinese Academy of Sciences. e) SERS for COR detection. Reproduced with permission.122 Copyright 2013, Royal Society of Chemistry.
The polyA strand was hybridized with an inhibitor strand to form a duplex, effectively separating guanine-rich overhangs and preventing the generation of G-quadruplex (Figure 15c).118 Upon the introduction of COR molecules, the formation of A-COR-A duplexes brought G-rich domains into close proximity. In the presence of K+ and the hemin cofactor, the G-rich overhangs formed hemin/G-quadruplex, which is a horseradish peroxidase (HRP) mimicking DNAzyme and catalyses the oxidation of ABTS2− (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) into colored ABTS⋅−, serving as a readout signal for COR detection.119 Additionally, a universal colorimetric detection platform based on graphene and DNA probes has been reported for various targets detection, including COR.120
A SPR based method for sensitive COR detection was developed,121 employing an Au film and AuNPs functionalized with polyA strands. In the presence of COR, the formation of A-COR-A duplex immobilized AuNPs on Au electrode (Figure 15d), which significantly enhanced the SPR signal through the localized electronic coupling between the AuNPs and Au electrode. Also, SERS was applied for label-free COR detection (Figure 15e).122 The binding of COR to polyA induced conformational transitions from single polyA into A-COR-A duplex were followed by the Raman intensity ratio of I(736 cm−1)/I(1319 cm−1) in the COR concentration range of 0.1 to 10 μM. Additionally, based on surface properties changes including mass, thickness, and refractive index (RI), DPI was employed for COR detection.123 Poly(ethylenimine) (PEI) was first adsorbed onto a silicon oxynitride chip, and single polyA strands (A48) formed a flat monolayer on PEI. Upon the introduction of COR, the conformational transitions from single strands to A-COR-A duplexes served for qualitative and quantitative COR analysis. The association rate constant (ka), dissociation rate constant (kd), and association constant (KA) between COR and A48 were measured as 4.95×103 M−1 s−1, 0.031 s−1, and 1.6×105 M−1, respectively. The detection limits of COR were 0.22 μM, 0.14 μM, and 0.32 μM based on mass, thickness, and RI calibration, respectively. The DPI-based method also demonstrated high COR selectivity against ethidium bromide, daunomycin, and methylene blue. Moreover, electrochemical detection of COR facilitated by exonuclease and nicking endonuclease was reported, revealing a linear COR detection range from 0.1 to 100 nM with a detection limit of 0.07 nM.124
The DNA templated AgNCs exhibited minimal fluorescence, which was significantly enhanced when G-rich overhangs were brought into close proximity.125 Leveraging this property, a fluorescence turn-on strategy was designed for COR detection. In the absence of COR, low fluorescence of AgNCs was observed (Figure 16a). However, in the presence of COR, the formed A-COR-A duplexes brought G-rich overhangs and AgNCs into close contact, resulting in a bright red fluorescence.126 A further COR detection method utilizing AgNCs and exonuclease III to facilitate fluorescence was realized.127 Additionally, the formed A-COR-A duplex was resistant to enzyme cleavage, such as exonuclease I (Exo I), the stable structure associated to SYBR Green I (SG I) yielding high fluorescence (Figure 16b).128 Other fluorescent COR assays include a polyA-graphene oxide platform,129 an Ag@SiO2-DNA-fluorophore nanostructure,130 and G-quadruplex based assays.131
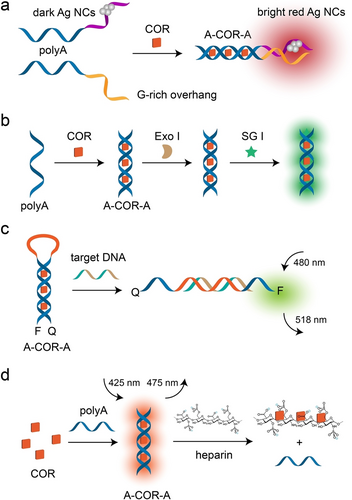
a) AgNCs based fluorescence detection of COR via A-COR-A induced proximity between AgNCs and G-rich overhangs. Reproduced with permission.126 Copyright 2015, Elsevier. b) Enzyme facilitated fluorescence analysis of COR. Reproduced with permission.128 Copyright 2013, Royal Society of Chemistry. c) A-COR-A duplex mediated MB as functional framework for fluorescence turn-on detection of target DNA. Reproduced with permission.132 Copyright 2012, Royal Society of Chemistry. d) A-COR-A guided fluorescence turn-off detection of heparin. Reproduced with permission.134 Copyright 2014, Elsevier.
Beyond detecting COR, the structural transitions induced by the A-COR-A duplex were also utilized to detect other analytes. For example, a molecular beacon (MB) structure was modified with carboxyfluorescein (FAM) as a fluorophore at the 5’-end and a 4-([4-(dimethylamino)phenoyl]azo)benzoic acid (DABCYL) as a quencher at the 3’-end, and the stem–loop MB configuration was bridged by the A-COR-A duplex, yielding a quenched fluorescence assembly (Figure 16c).132 A target DNA hybridized with the loop domain, separated the A-COR-A duplex and restored the fluorescence of FAM. A similar method was reported for detecting heparin in plasma.133
An alternative method applying label-free heparin detection utilizing COR as a fluorescent indicator was realized (Figure 16d).134 In the hydrophobic environment of the A-COR-A duplex conformation, COR exhibited high fluorescence compared to its free state in solution. Introduction of heparin resulted in the formation of an energetically favourable heparin-COR complex via electrostatic heparin promoted dimerization of COR, leading to the disassembly of A-COR-A duplex. Consequently, the COR fluorescence served as a turn-off signal for detecting heparin, revealing high selectivity (>90 fold) against hyaluronic acid and chondroitin sulfate. Using a single FAM modified strand, selective fluorescent detection of both COR and heparin was achieved.135 The DNA probe was modified with FAM at 5’-end and its 3’-terminal was designed to be G-rich domain. Formation of the A-COR-A duplex resulted in the fold-back of the probe and close contact between FAM and G-rich domain, resulting in FAM fluorescence quenching via photoinduced electron transfer. In the presence of heparin, COR preferentially bound to heparin, leading to the separation of the A-COR-A duplex and the restoration of FAM fluorescence. Therefore, detection of COR and heparin was achieved by fluorescence turn-off and turn-on of FAM, respectively. Other examples include COR and SG I facilitated detection of heparin,136 and polymerase extension using SG I for detecting COR and heparin.137 Additionally, other targets, such as over-sulfated chondroitin sulfate,138, 139 miRNA,140 melamine,141 cyclic diadenosine monophosphate,142, 143 and cell apoptosis,144 were successfully detected via COR-induced A-COR-A duplex structural transitions.
5 T ⋅ A-T Triplex
DNA triplexes, such as T ⋅ A-T or C+ ⋅ G-C, arise from the binding of triplex-forming oligonucleotides (TFOs) to the polypurine strand in the major groove of duplex through Hoogsteen interactions. Protonation of polyT or polyC TFOs is essential for facilitating their binding to the polypurine strands. For example, N3 protonation of cytosine (pKa 6.5) in polyC TFOs forms Hoogsteen bonds with N7 of guanine. Consequently, the formation/dissociation of triplex structures is governed by the system pH induced protonation/deprotonation, respectively. The pH-responsive ranges of these structures are finely tuned by the content of T ⋅ A-T or C+ ⋅ G-C units in the DNA sequences. For example, the pH-dependence of a DNA nanoswitch spanning a 5 pH unit range was achieved by reducing the T ⋅ A-T content from 100 % to 50 %.145 The pH-responsive properties and diverse applications of DNA triplexes for sensing, nanoassembly, molecular switches, catalysis, and cargo carriers have been comprehensively reviewed.4, 146 Recently, applications of these triplex structures included their use as nanosensors in lysosomes,147, 148 plasmonic assemblies,149-152 soft matter,153 computation,154, 155 structural analysis,156-160 DNA crystals,161 DNA nanoswitch,162-169 DNA-templated circularly polarized luminescence,170 DNA origami and machine,171-176 biosensing,58, 177, 178 colorimetry,179 tracking endosomal escape,180 circulating tumor cells fishing,181 synthetic nucleic acid receptors,182 and more. Many of these applications involve triplex structures containing a mixture of T ⋅ A-T and C+ ⋅ G-C triplets. In addition, DNA : RNA triplex and pure RNA triplex structures have also been reported,183-185 shedding light on their biological functions.186, 187 However, due to the scope of this review, these studies will not be elaborated upon, as the focus is primarily on the T ⋅ A-T triplexes and their applications.
5.1 pH-Triggered Structural Characteristics of T ⋅ A-T Triplex
In the T ⋅ A-T triplex conformation, polyT binds parallelly to the polyA strand of the A-T duplex via Hoogsteen interactions under neutral pH conditions (Figure 17a). Under mild alkaline conditions, the triplex dissociates into an A-T duplex and a single-stranded polyT domain due to the deprotonation of thymines (pKa 10),145 Figure 17b. These pH-triggered assembly/disassembly processes are reversible. The CD spectra of the T ⋅ A-T triplex at pH 7.2 and its dissociation into polyT and A-T duplex at pH 10.2 are depicted in Figure 17c, indicating a noticeable increase in band intensity at 220 nm.18 Additionally, UV-melting profiles of the T ⋅ A-T triplex at neutral pH reveal two melting processes, corresponding to the dissociations of T ⋅ A-T triplex and A-T duplex, with Tm values of 32 °C and 60 °C, respectively.90 In addition to intermolecular T ⋅ A-T triplex, intramolecular triplex structures folded from a single oligo strand have been studied, driven by a significant and favourable enthalpic contribution.188 The effects of various factors, including loop length and sequence, pH, as well as ions, on the thermodynamic stability of T ⋅ A-T triplexes were studied.188-190 For example, sodium ions and cytosines protonation in the loop have been shown to stabilize triplex structures.
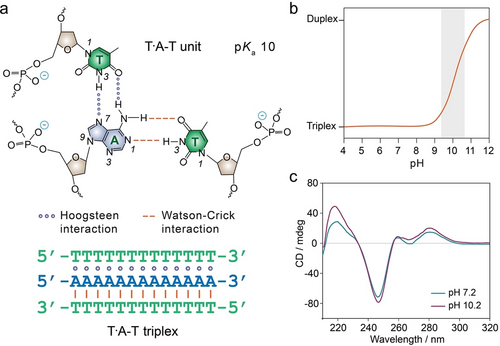
T ⋅ A-T triplex structure. a) Chemical structures of T ⋅ A-T unit crosslinked by Hoogsteen interaction and Watson–Crick interactions. b) T ⋅ A-T triplexes disassemble into an A-T duplex and polyT single strand at high pH due to the deprotonation of thymines (pKa 10). Reproduced with permission.145 Copyright 2014, American Chemical Society. c) CD spectra of T ⋅ A-T triplexes formed at pH 7.2 and their dissociation at pH 10.2. Reproduced with permission.18 Copyright 2023, Wiley-VCH GmbH.
5.2 T ⋅ A-T Triplex-Based DNA Hydrogels
The dynamic pH-induced T ⋅ A-T triplex reconfiguration to construct stimuli-responsive DNA hydrogels for controlled drug/payloads release, shape-memory, shape modulation and self-healing applications have been developed.90, 153, 191-196 For example, adding polyA strands into polyT based copolymer solution at pH 7.0 yielded a DNA hydrogel crosslinked by T ⋅ A-T triplex units. Adjusting the system pH to 10.0 resulted in a transition to liquid state due to the separation of T ⋅ A-T triplex bridges (Figure 18a).90 The reversible transitions between the hydrogel state and the liquid state were controlled by the system's pH values. Furthermore, the hydrogel was loaded with a payload, such as COR, which preferentially bound to T ⋅ A-T triplexes at pH 7.0. Controlled release of the payload was demonstrated by adjusting the pH value to 10.0. Additionally, the binding of COR to T ⋅ A-T triplexes within the DNA hydrogel enhanced its rigidity, as evidenced by an increase of G’ value from 48 Pa to 64 Pa.
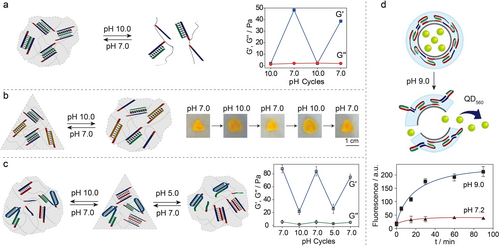
a) Reversible formation/dissociation of T ⋅ A-T triplex crosslinked DNA hydrogel at pH 7.0 and pH 10.0, respectively. Reproduced with permission.90 Copyright 2015, Royal Society of Chemistry. b) A shape-memory DNA hydrogel with T ⋅ A-T triplex as the temporary memory element and hybridized duplex as the permanent memory element exhibiting reversible shape transitions between the triangle and the shapeless state upon pH triggers. Reproduced with permission.192 Copyright 2015, Wiley-VCH GmbH. c) A shape-memory DNA hydrogel with two internal memories mediated by triplexes configurations. Reproduced with permission.191 Copyright 2016, Wiley-VCH GmbH. d) A DNA hydrogel microcapsule for pH-triggered release of payloads (QD560).195 Copyright 2016, American Chemical Society.
Incorporating hybridized DNA duplexes as permanent crosslinkers into a hydrogel system enabled the construction of a shape-memory DNA hydrogel, with T ⋅ A-T triplexes serving as the temporary memory elements.192 At pH 7.0, the DNA hydrogel was cooperatively stabilized by duplex and T ⋅ A-T triplex, maintaining a rigid triangle shape (Figure 18b). Adjusting the system pH to 10.0 resulted in the dissociation of T ⋅ A-T triplex bridges, leading to a shapeless quasi-liquid state crosslinked solely by the permanent duplex structure. Restoration into the triangle-shaped DNA hydrogel state was achieved upon adjusting the pH value to 7.0, which prompted the regeneration of T ⋅ A-T triplex bridges. The reversible transitions between the triangle shape and the shapeless quasi-liquid state were facilitated by switching the systemic pH values between 7.0 and 10.0, respectively.
Expanding beyond non-stimuli-responsive DNA duplexes as permanent memory elements, T ⋅ A-T triplex can function as both pH-responsive crosslinkers and temporally permanent bridges. Through strategic integration of two pH-responsive triplexes, T ⋅ A-T and C+ ⋅ G-C, a DNA hydrogel exhibiting two internal memories was developed.191 At pH 7.0, the DNA hydrogel was stabilized by both T ⋅ A-T triplex and G-C rich hybridized duplexes, presenting a rigid triangular shape crosslinked by two bridging motives (Figure 18c). Adjusting the system pH value to 10.0 led to the dissociation of T ⋅ A-T triplexes, resulting in a shapeless quasi-liquid hydrogel state bridged by the G-C rich duplexes acting as memory units. Restoration of the triangular shape occurred upon reverting the pH value to 7.0, which regenerated the T ⋅ A-T triplex bridges. When the system was adjusted to pH 5.0, T ⋅ A-T triplex structures remained intact due to the protonation of polyT TFOs, while the G-C rich duplex transitioned to C+ ⋅ G-C triplex due to the protonation of polyC TFOs. Similarly, a shapeless quasi-liquid state was achieved at pH 5.0, with T ⋅ A-T triplex serving as the temporally permanent memory bridges. Restoration of the triangular shape occurred upon reverting the pH value to 7.0. The pH-triggered reversible transitions between the triangular shape and the shapeless quasi-liquid state were characterized by rheology measurements, where G’ values of ∼80 Pa and ∼20 Pa correspond to the two different states, respectively (right panel, Figure 18c).
A similar strategy was employed to create a pH-controlled bidirectionally pure DNA hydrogel, which maintained a hydrogel state at pH 7.0 and transitioned into a liquid state at either pH 5.0 or pH 10.0.196 Additionally, DNA hydrogels utilizing T ⋅ A-T triplexes as the permanent memory elements and other configurations, such as i-motif and G-quadruplex, as temporary memory elements, exhibited stimuli-responsive reversible bending properties, which were induced by internal stress changes upon the formation/dissociation of functional DNA structures.193
Moreover, enzymes were integrated in T ⋅ A-T triplex crosslinked hydrogels and biocatalysed pH-changes in the gels were used to develop self-healing matrices.194
Harnessing the advantageous properties of external stimuli responsiveness, the controlled payloads delivery and release represent an important medical application of DNA hydrogels. Downsizing bulky DNA hydrogels into micro/nano scale structures, known as DNA microgels/nanogels, facilitates targeted payloads delivery.36, 197 For example, pH-responsive DNA hydrogel microcapsules were constructed utilizing T ⋅ A-T triplexes as crosslinker.195 Subjecting the microcapsules to pH 9.0 separated the T ⋅ A-T triplex units, resulting in the disassembly of the DNA hydrogel microcapsules and the subsequent release of quantum dots (QD560) (Figure 18d).
5.3 Constitutional Dynamic Networks
Taking inspiration from biological systems and living organisms operating complex network exhibiting dynamic and adaptive features, artificial CDNs have been developed.198-200 These networks consist of a mixture of molecular or macromolecular constituents that reveal interconversion exchange of constituents dictated by their thermodynamic stabilities. Leveraging the reconfiguration features of DNA structures, stimuli-responsive DNA CDNs have been systematically demonstrated.201 These include consecutive feedback,202 DNA-tweezer machines,203 gene expression circuits,204 biocatalytic cascades,205, 206 evolutionary principles,207 DNAzyme catalysis,208 logic gates,209 catalytic functions,210, 211 loads release,212 DNA replication machineries,213 hydrogels,214 aggregation of nanoparticles (Au or QDs),215 among others.
Among these CDNs, T ⋅ A-T triplex structures serve as structural motifs to regulate the energetic stabilization of the constituents. As depicted in Figure 19a, the CDN K comprises four interconvertible and mechanically triggered equilibrated DNA tweezers (AA’, BB’, AB’ and BA’), existing in closed and open configurations, respectively. Subjecting the CDN K system to the external effector (E), a polyA strand, led to the formation of T ⋅ A-T triplex stabilized AB’-T constituent,203 resulting in the dynamic transition of CDN K to CND L, where constituents AB’-T and BA’ are upregulated and AA’ and BB’ are downregulated, as evidenced by the decreased signals of colorimetric ABTS⋅− (ABTS2− catalyzed by hemin/G-quadruplex DNAzyme (domains a and a’) in the presence of H2O2, panel i) and fluorescent FAM (generated by Mg2+-dependent DNAzyme, panel ii), respectively. Subjecting the CND L to the counter-effector E’ (a polyT strand complementary to E) resulted in the displacement of E and the separation of the T ⋅ A-T triplex structures, which led to the transition from CDN L to CDN K. Therefore, by the cyclic treatment of CDNs with E and E’, the CDN could be switched between CDN L and K, respectively, illustrating the control over the mechanical constituents in the systems and their chemical functionalities.
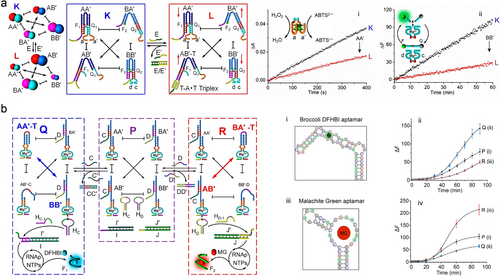
a) Schematic presentation of CDNs consisting of DNA tweezer machines. Formation of T ⋅ A-T triplex induced downregulation of constituent AA’ and BB’ from CDN K to L was monitored by the absorbance of colored ABTS⋅− (panel i) and the fluorescence of DNAzyme cleaved substrate (panel ii), respectively. Reproduced with permission.203 Copyright 2017, American Chemical Society. b) Schematic presentation of the triggered, T ⋅ A-T triplex stabilized CDN-guided transcription of mRNAs aptamers for DFHBI (panel i and ii) and malachite green (panel iii and iv). The introduction of strand C’ into CDN P resulted in upregulated T ⋅ A-T triplex stabilized constituent AA’-T and BB’, leading to CDN Q, which subsequently generated RNA aptamer for DFHBI. Similarly, the introduction of strand D’ into CDN P resulted in upregulated T ⋅ A-T triplex stabilized constituent BA’-T and AB’, leading to CDN R, which subsequently generated RNA aptamer for MG. Reproduced with permission.204 Copyright 2020, American Chemical Society.
In another study, CDNs as functional modules mimicking natural circuits was demonstrated by CDNs-guided synthesis of genes, controlled transcription of RNAs, and dictated transcription/translation synthesis of proteins,204 Figure 19b. A reaction module parent CDN P composed of four dynamically equilibrated constituents AA’, AB’, BA’, and BB’, and auxiliary two hairpins, HC and HD, and two templates, I/I’ and J/J’, was constructed. Subjecting the reaction module to an auxiliary strand, C’, led to the displacement of strand C from constituent AA’ and to the generation of an intramolecular T ⋅ A-T triplex stabilized constituent AA’, resulting in upregulated constituent AA’-T, as well as BB’. Conversely, constituents AB’ and BA’ were downregulated, yielding a compositionally reconfigured CDN Q. Constituent BB’ was engineered to cleave hairpin HC the Mg2+-dependent DNAzyme tethered to constituent BB’ and generate the cleaved fragment HC−1, hybridized with template I/I’, resulting in an active transcription template yielding in the presence of RNA polymerase (RNAp) and a mixture of NTPs the transcription of the broccoli DFHBI ((5Z)-5-[(3,5-difluoro-4-hydroxyphenyl)methylene]-3,5-dihydro-2,3-dimethyl-4H-imidazol-4-one) aptamer. Formation of the DFHBI/I’ aptamer complex (panel i) activated the fluorescence of DFHBI at λem=500 nm, and the time-dependent fluorescence profiles provided a readout signal for the RNA aptamer (panel ii). Similarly, the effector, D’, triggered the transition of parent CDN P into R, yielding constituent BA’-T (stabilized by the intramolecular T ⋅ A-T triplex). This behavior led to the upregulation of AB’, while orthogonal downregulation of the constituents AA’ and BB’. Subsequently, hairpin HD was cleaved by constituent AB’, yielding fragment HD-1 hybridized with template J/J’, yielding the transcription factors for the RNAp/NTPs transcription of the Malachite Green (MG) RNA aptamer (panel iii). The resulting fluorescence of MG-aptamer at λem= 655 nm provided a dynamic readout signal for temporal operation of the transcription machinery (panel iv).
Furthermore, nucleic acid-based CDNs conjugated to enzymes were implemented for the CDN-guided, triplex-triggered, control over biocatalytic cascades.205 This is exemplified in Figure 20a with the dynamic control of the GOx/HRP cascade. A CDN M composed of four constituents AA’, BB’, AB’ and BA’, where each of the constituents was modified with a Mg2+-dependent DNAzyme as concentration transducing unit, panel i, and a bi-loop domain enabling the formation of a T ⋅ A-T triplex was assembled. The components of constituent AA’ were functionalized with GOx/HRP biocatalysts allowing the operation of the GOx/HRP biocatalytic cascade within the confined constituent structure, where the aerobic oxidation of glucose yields gluconic acid and H2O2, followed by the H2O2 oxidation of ABTS2− to ABTS⋅−, panel ii. The T1-triggered stabilization of constituent AA’ through formation of the T ⋅ A-T triplex reconfigures CDN M into CDN X, where AA’ and BB’ are upregulated and BA’ and AB’ are downregulated. In contrast, the T2-triggered stabilization of constituent BA’, through formation of the triplex T ⋅ A-T in the bi-loop domain, results in the reconfiguration of CDN M into CDN Y, where constituents BA’ and AB’ are upregulated and constituents AA’/BB’ are downregulated. The dynamic concentration changes of the constituents upon the T1 or T2-triggered reconfiguration of CDN M or CDN X or CDN Y are displayed in panels iii–vi. The upregulation of constituent AA’ in CDN X leads to the upregulation of the GOx/HRP biocatalytic cascade, as compared to the biocatalytic cascade operating in CDN M, whereas the T2-stimulated downregulation of AA’, is accompanied by the downregulation of the GOx/HRP cascade, as compared to the biocatalytic cascade proceeding in CDN M, Figure 20b.
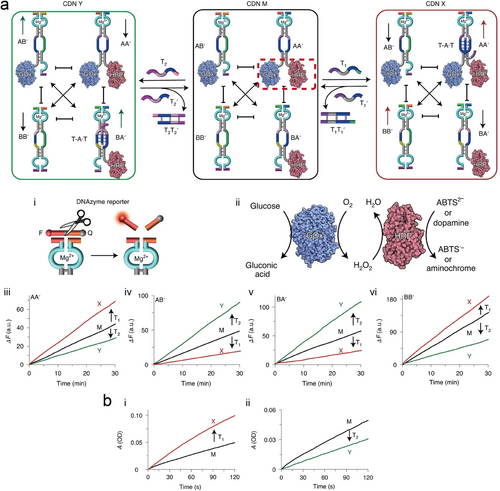
Schematic presentation of nucleic acid–enzyme conjugates based CDNs for triggered, network-driven, biocatalytic cascades. a) Introduction of effector T1 into CDN M resulted in the T ⋅ A-T triplex stabilized upregulated constituent GOx/HRP-AA’ and BB’, leading to CDN X. Introduction of effector T2 into CDN M resulted in the T ⋅ A-T triplex stabilized upregulated constituent HRP-BA’ and GOx-AB’, leading to CDN Y. The readout signals were monitored by a Mg2+-dependent DNAzyme (panel i) or GOx/HRP enzyme cascade reactions activated the oxidation of ABTS2− or dopamine (panel ii). The up or downregulated constituents AA’, AB’, BA’, and BB’ are shown as panel iii, iv, v, and vi, respectively. b) Time-dependent absorbance changes of ABTS⋅− on the T1-stimulated reconfiguration of CDN M into CDN X and the accompanying upregulation of the GOx/HRP biocatalytic cascade (panel i), as well as T2-stimulated reconfiguration of CDN M into CDN Y and the accompanying downregulation of the GOx/HRP cascade (panel ii). Reproduced with permission.205 Copyright 2020, Springer Nature Limited.
Additional T ⋅ A-T triplex regulated CDNs have been designed for various applications, including the integration of photocatalytic and dark-operating catalytic biomimetic transformations,216 intercommunication,217 regulation of the catalytic functions of DNAzymes,218 as well as light-induced reversible reconfiguration and switchable catalysis.219
5.4 Dynamic Reconfigurable Hydrogel Materials
Triplex T ⋅ A-T triggered crosslinked, dynamic CDNs revealing stiffness-dictated hydrogel functions were demonstrated,214, 220 Figure 21. Functional constituents consisting of Y-shaped bi-loop engineered nucleic acid structures AA’ and BB’ and bis-Y-shaped interlinked structure composed of AB’ and BA’ provided the subunits for the assembly of the crosslinked hydrogel framework. The arms associated with constituents included toehold single-stranded strands exhibiting base-complementarities guiding the dynamic crosslinking of the dynamically equilibrated constituents into a 3D hydrogel X, Figure 21a. The stiffness of the resulting hydrogel is controlled by the degree of crosslinking of the Y-shaped constituents AA’/BB’ by the bis-functional crosslinkers AB’/BA’. Stabilizing the bi-loop domain of BA’ with the triggering strand E1, generating a T ⋅ A-T complex reconfigures the hydrogel X into hydrogel Y, where the degree of crosslinking of hydrogel framework increased, resulting in a hydrogel of enhanced stiffness (Young's module Yeff=23.45±0.43 kPa) as compared to the hydrogel X (Yeff=13.18±0.29 kPa). Alternately, subjecting the hydrogel X to the strand E2, stabilizing the bi-loop domain of BB by a T ⋅ A-T triplex, dynamically reconfigures hydrogel X into hydrogel Z where the Y-shaped constituents AA’ and BB’ are enriched on the expense of a lower degree of crosslinking by BA’/AB’. This results in a hydrogel of lower stiffness in state Z, Yeff=3.75±0.03 kPa, as compared to hydrogel X, Figure 21b. By applying appropriate counter triggers, E1’ and E2’, the respective T ⋅ A-T complexes were separated, resulting in the recovery of the medium stiffness hydrogel in state X. The control over the stiffness of the hydrogel framework by appropriate T ⋅ A-T complexes was implemented for different applications. For example, treatment of physically interconnected hydrogel pieces revealing medium stiffness in state X with the trigger E1 resulted in the higher stiffness hydrogel and to high stiffness interlinked boundary yielding a “healed” integrated hydrogel material, Figure 21c. Also, encapsulation of the chemotherapeutic anti-cancer drug, DOX, into the medium-stiffness hydrogel X resulted in the E2-triggered release of the drug upon reconfiguration to the lower stiffness hydrogel Z, Figure 21d, panel i. Accordingly, subjecting of MDA-MB-231 breast cancer cells to the hydrogel X triggered by E2 led to >90 % of cell death after three days of treatment, panel ii.
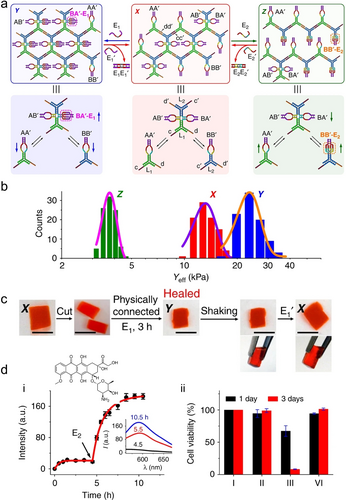
a) Schematic design of nucleic acids-triggered all-DNA hydrogel revealing reversible and switchable stiffness properties guided by CDNs. b) Histograms and corresponding Gaussian fits of Young's moduli associated with CDN hydrogels X, Y, and Z. c) Self-healing of CDN hydrogel X. d) Time-dependent release profile of DOX from hydrogel X (panel i) and the cytotoxicity of the released DOX from hydrogel X without or with trigger E2 toward MDA-MB-231 breast cancer cells. Reproduced with permission.214 Copyright 2019, Springer Nature Limited.
6 A Comparison of polyA Derived Noncanonical DNA Structures
The previous sections summarized the pH-triggered dynamic structural transitions of various polyA derived noncanonical DNA structures, including A-motif duplex, A-CA triplex, A-COR-A duplex, and T ⋅ A-T triplex. Their assembly/disassembly processes are achieved by varying system pH values. For comparison, their pH-triggered dynamic structural transitions within a pH range from 1.0 to 10.0 are demonstrated in Figure 22. Parallel A-motif duplex, crosslinked by AH+-H+A units, is generated under highly acidic conditions (e.g., pH 1.0) due to the protonation of adenine nucleotides (pKa 3.5) and disassembles at pH 5.0 driven by the deprotonation. Upon the protonation of CA cofactors (pKa 6.9), parallel A-CA triplex forms at pH 5.0 and dissociates at pH 7.0 upon deprotonation. While COR-mediated antiparallel A-COR-A duplex is stable at pH 7.0, it separates into its constituents at pH 5.0 ascribed to the low stability. The T ⋅ A-T triplex assembles at pH 7.0 upon the protonation of polyT strand (pKa 10.0) and separates into A-T duplex and polyT strand at pH 10.0. These dynamically configurational transitions are pH-reversible, which could be achieved by recycling system pH values via direct pH-adjustment using acid/base or biocatalytic reactions, such as glucose catalysis by GOx to gluconic acid, urea hydrolysis by urease forming ammonium ion, and acetylcholine hydrolysis by AChE generating choline and acetic acid. Considering the structural characteristics and pH-responsiveness of these polyA derived noncanonical DNA conformations, more sophisticated and pH-cascaded developments, such as dynamic molecular switches, assembly of nanoobjects, computing and logic gates, as well as smart carriers for sequential cargo delivery, could be envisaged.
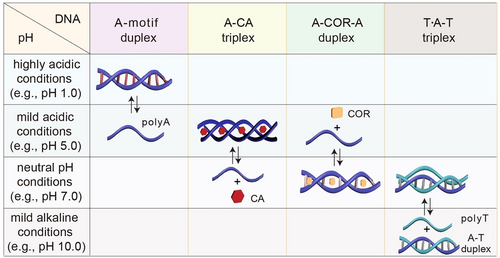
A summary and comparison of polyA derived noncanonical DNA structures and their pH-triggered structural transactions.
7 Application of pH-Cascaded Structural Transformations
Cascade reactions, in which consecutive chemical transformations or processes take place to achieve rapid construction of molecular complexity, have been widely utilized in biosynthesis or total synthesis.221, 222 Recently, DNA mediated cascade reactions have also emerged as a promising area of research.223, 224 In contrast to single pH stimulus, pH-cascaded dynamic structural transitions represent an advanced approach in materials science and engineering, demonstrating adaptivity to diverse environmental stimuli. Figure 23a introduces a pH-cascaded DNA hydrogel mediated by reconfigurable A-motif duplex, i-motif quadruplex, and T ⋅ A-T triplex conformations dynamically responded to sequential pH changes (1.1→5.2→7.2→10.2).18 A copolymer comprising a polyacrylamide backbone as well as polyA and ployC tethers existed in a liquid state at pH 7.2. Adjusting the system pH to 1.1 led to protonation of adenines, which resulted in the formation of A-motif duplex, acting as bridges to form 3D crosslinked copolymer networks (hydrogel state, panel i). When the pH value was adjusted to 5.2, deprotonation of adenine units separated the A-motif bridges, while protonating the cytosines leading to the formation of i-motif structures, which acted as the crosslinking units to retain the hydrogel state (panel ii). Subsequently, adjusting the pH to neutral (7.2) separated the i-motif structures, yet simultaneous introduction of auxiliary polyT21 and polyT45 strands, resulting in the generation of T ⋅ A-T triplexes retaining the hydrogel state (panel iii). Finally, at pH 10.2, deprotonation of thymines resulted in the disassembly of T ⋅ A-T triplex into A-T duplex and single polyT45 strands, corresponding to a liquid state (panel iv). The sequential addition of auxiliary strands polyT21 and polyT45 is critical for forming a mild alkaline pH responsive hydrogel, where the hydrogel-to-liquid transition occurs at pH 10.2. Rational design of these sequences and manipulation of their structures enabled the formation of DNA hydrogels with finely tuned pH-responsiveness.
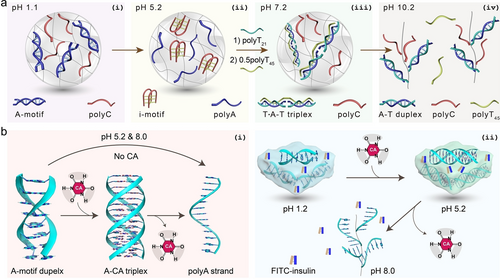
a) A pH-cascaded DNA hydrogel mediated by reconfigurable A-motif duplex, i-motif quadruplex, and T ⋅ A-T triplex structures exhibiting dynamic structural transitions. Hydrogel state retains at pH 1.1 (panel i), 5.2 (panel ii) and 7.2 (panel iii), while liquid state is observed at pH 10.2 (panel iv). Reproduced with permission.18 Copyright 2023, Wiley-VCH GmbH. b) A pH-cascaded DNA hydrogel mediated by reconfigurable A-motif duplex and A-CA triplex (panel i), in which FITC-insulin is encapsulated and its controlled release is achieved at physiological pH value (panel ii). Reproduced with permission.66 Copyright 2024, American Chemical Society.
By employing polyA and CA cofactors, another pH-cascaded DNA hydrogel mediated by A-motif duplex and A-CA triplex was demonstrated.66 As illustrated in panel i (Figure 23b), the hydrogel was initially stabilized by A-motif duplex at pH 1.2. Adjusting the system pH value to 5.2, the crosslinking units to maintain the hydrogel state switched to A-CA triplex structures due to the protonation of CA. The transition from hydrogel to liquid was achieved by changing the pH value to 8.0, leading to the deprotonation of CA cofactors. In the absence of CA, the hydrogel bridged by A-motif duplex at pH 1.2 transitioned to liquid state at both pH 5.2 and pH 8.0. FITC-insulin was encapsulated and protected in the DNA hydrogel under acidic conditions. At pH 8.0, the disassembly of hydrogel into liquid state was driven, facilitating the complete release of FITC-insulin. This study holds promise for future development of a pH-responsive platform for oral drug administration of diabetics, where the hydrogel provided drug protection in the stomach and duodenum before releasing the payload in the small intestine.
Moreover, advancements have been demonstrated with the development of pH-cascaded A-motif and A-CA triplex mediated DNA fibres.65 These fibres were crafted from polyA strands comprising 30 adenines, which self-assembled into triplex oligomers upon pairing with CA cofactors. Subsequently, these triplex oligomers merged into one-dimensional fibres through a static manner, resulting in fibres with intermittent gaps (Figure 24a and 24b). To correct these gaps and promote their hierarchical assembly into nanocable architectures with enhanced thermal stability, a novel oscillatory proton release and dissipation system was implemented. The system orchestrated pH oscillations among three distinct, transient states—A-CA triplex, A-motif duplex, and single-stranded polyA—via a recyclable, out-of-equilibrium process. This mechanism relied on a merocyanine photoacid (MEH) that, upon exposure to broad-spectrum visible light, liberated protons to transform into a spiropyran (SP), thereby altering the pH of a saturated solution from 5.3 to 3.5 (buffer was not employed). During time-intervals of darkness, the protons were reabsorbed by the photoacid. This dissipative pathway promoted the directed assembly of polyA monomers with CA cofactors into nanocable superstructures (Figure 24c). This study introduced a chemical pathway for error correction within the fibre structures, distinct from conventional thermal annealing processes, thereby enhancing the morphology and property of supramolecular materials through the utilization of, transient, out-of-equilibrium systems.
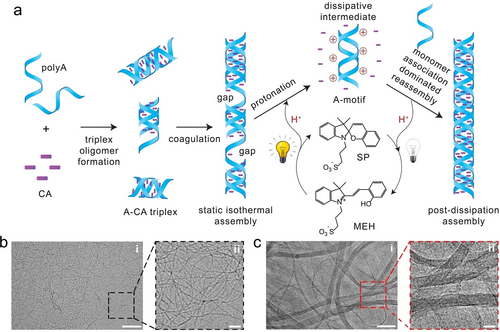
a) A light-driven cycle of proton dissipation to mediate the assembly of DNA supramolecular structures, transforming intermittent-gapped fibres into perfect nanocables. Visible light exposure triggers the liberation of protons from MEH, disassembling the notched fibres and forming dissipative A-motif structures. Subsequent proton dissipation, following light cessation, facilitates slow monomer reassociation into nanocable structures. Cryo-electron microscopy (cryo-EM) images of DNA fibres before (b) and after (c) proton dissipation. Scale bars, 100 nm (panel i) and 25 nm (panel ii). Reproduced with permission.65 Copyright 2021, Springer Nature Limited.
8 Summary and Outlook
Noncanonical DNA structures, including i-motif, G-quadruplex and triplex, have been extensively studied and applied in nanotechnology, biology, materials science, and engineering.4, 8, 225 Ongoing scientific endeavors continue to focus on the exploration, development, and utilization of novel noncanonical DNA configurations. The present review highlighted recent advancements in polyA-derived noncanonical DNA structures, including A-motif duplex, A-CA triplex, A-COR-A duplex, and T ⋅ A-T triplex. Structural and mechanistic characteristics of these configurations triggered by external stimuli, such as pH values (protonation/deprotonation), or low-molecular-weight cofactors (CA, COR), have been elucidated. Reversible conformational transitions between single-stranded polyA, duplex (A-motif, A-COR-A), and triplex (A-CA, T ⋅ A-T) upon trigger/counter-trigger were discussed. Diverse applications of these unique configurations were highlighted, including pH-responsive DNA hydrogels for drug delivery, shape memory and self-healing; small molecule-mediated electronic devices; pH-facilitated convenient anchoring of DNA strands onto nanoparticles; monitoring of biocatalytic reactions by enzymes; controlled assembly of micro-fibres; sensitive (bio)sensing of various targets; construction of orthogonal constitutional dynamic networks and switchable DNA machineries. Moreover, a comparison between these polyA-driven DNA conformations was provided. By strategically integrating these structural characteristics and their responsiveness to external stimuli, advanced systems, such as pH-cascaded DNA hydrogels and supramolecular fibres adaptable to environmental triggers with dynamic physiochemical properties changes, have been addressed.
With these significant achievements in poyA-derived noncanonical DNA configurations, challenges remain. For example, due to the homogeneity of a single polyA strand, undesired secondary structures, such as foldbacks, may form, which can interfere with the physicochemical properties of the desired configuration. The length of polyA strands should be carefully considered when designing sequences for specific applications. Moreover, potential degradation by nucleases should also be taken into account, especially for RNA secondary structures derived from polyA sequences. Applying multiple polyA-derived secondary structures within a single system presents additional challenges, requiring careful design to achieve the desired functionalities. Nonetheless, future promising developments can be envisaged:
-
Therapeutics encapsulation and targeted delivery. Targeted therapeutics delivery through stimuli-responsive DNA hydrogels is a significant biomedical application requiring controlled release to achieve desired therapies. For example, pH-cascaded DNA hydrogels with adaptive capabilities to environmental conditions hold great promise for oral drug delivery, including insulin or mRNA vaccines. By rationally designing DNA hydrogel structures and properties to dynamically respond to gastrointestinal tract stimuli (such as pH, enzymes, low-molecular-weight ligands), precision therapies are achievable. While DNA strands are generally considered biocompatible and biodegradable, the currently adopted monomers, such as acrylamide, used to generate polymer backbones for generating hydrogel matrices, are toxic. Natural biopolymers, especially polysaccharides like dextran, cellulose, and chitosan, offer promising alternatives.
-
Minimized intelligent platforms. Promising biomedical applications involve, however, targeted drug delivery in cells or tumor microenvironments, which necessitates the miniaturization of current bulky delivery platforms. Further development of polyA-driven DNA micro/nanogels or capsules with smart sensing and adaptive responsiveness to intracellular components or biomarkers in the microenvironments is essential. Additionally, mimicking cellular compartment interactions or cell-cell communications through trigger-cascaded architectures like nanogel-in-nanogel or nanocapsule-in-nanocapsule or nanogel-in-nanocapsule may provide in situ therapeutics or bioimaging reagents. Nevertheless, the immunogenicity of these carriers should be considered. While polyA is a tail component of mRNA in all eukaryotic cells, many other non-polyA DNA strands may also be used as constitution carriers and biosafety of the systems should be addressed.
-
Supramolecular electronics. Beyond current applications, further exploration of the potentials of polyA-based noncanonical DNA structures in other research fields is anticipated, such as single supramolecular electronics with dynamic structural transitions adaptive to environmental cues. As per Moore's law, silicon-based electronic components are approaching their minimum size limit of several nanometers.226 These size-/physiochemical property-controllable DNA configurations derived from polyA may serve as feasible, bottom-up, alternatives to silicon circuit elements.
-
Novel noncanonical frameworks. Detailed investigations into the structural characteristics of polyA-driven secondary structures compared to B-DNA conformations and other noncanonical configurations may offer additional insights into their structure, electronic conductance/charge transport, etc., thereby providing guidance for their rational applications. Additionally, the introduction of low-molecular-weight cofactors into noncanonical DNA configurations to finely tune their physiochemical properties, such as stability, conductivity, rigidity, and fluorescence are interesting paths to follow. Further exploration of the interaction mechanisms, such as intercalation, π–π stacking, and electrostatic attraction, between cofactors and noncanonical structures needs to be addressed.
-
Artificial intelligence (AI) boosting new developments. In addition to the currently reported noncanonical DNA configurations derived from polyA, further investigations into novel polyA secondary structures triggered by external stimuli are necessary. Experimental and theoretical studies in combination with AI to predict and validate novel noncanonical DNA structures, beyond those based solely on polyA, may be beneficial. This endeavor requires collaborative and sustained efforts from chemists, physicists, biologists, computer scientists, AI engineers, and other relevant disciplines.
-
Noncanonical RNA structures. The exploration of polyA-derived secondary RNA structures and their functions in gene regulation and protein translation is critical in biology. For instance, a self-assembled RNA triplex hydrogels actively participated in silencing an oncogene, resulting in significant tumor shrinkage (90 %).185 Additionally, a G-quadruplex based RNA hydrogel exhibited catalytic activity and enhanced protein expression in sub-compartmentalized, phase-separated translation environments.227 These preliminary reports highlight the high potential of noncanonical RNA structures in future nanomedicine for treating various diseases. Moreover, the formation of secondary RNA configurations might inhibit their cleavage by nucleases, thereby increasing their physiochemical stability both in vitro and in vivo.
Acknowledgments
The study is supported by Career Development Fund (C210112014) and SERC Central Research Fund (CRF, UIBR, KIMR220901aSERCRF) (Y. H.), IMRE, A*STAR, Singapore, as well as the Israel Science Foundation, ISF (I. W.) and the Minerva Center for Biohybrid Complex Systems, The Hebrew University of Jerusalem.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Biographical Information
Yuwei Hu completed his Ph.D. studies jointly at Jilin University and the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences under the supervision of Prof. Li Niu. He conducted postdoctoral research with Prof. Itamar Willner at The Hebrew University of Jerusalem. He is a Senior Scientist at the Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), Singapore. His research interests include smart multi-functional materials, sustainable bioplastics, stimuli-responsive DNA hydrogels, DNA nanotechnology, therapeutics delivery, and materials for biosensing.
Biographical Information
Itamar Willner completed his Ph.D. at The Hebrew University of Jerusalem in 1978. After postdoctoral research at UC Berkeley, he joined The Hebrew University of Jerusalem, where he has been Professor since 1986. His research interests include bioelectronics, nanobiotechnology, artificial photosynthesis, artificial enzymes, dynamic networks, and stimuli-responsive materials. He received the Israel Prize in Chemistry, the Rothschild Prize, the EMET Prize, and the Israel Chemical Society Gold Medal. He is a member of the Israel Academy of Sciences and Humanities, the German Academy of Sciences-Leopoldina, and the Chinese Academy of Sciences.






