Total Syntheses of Streptamidine and Klebsazolicin Using Biomimetic On-Resin Ring-Closing Amidine Formation
Abstract
Diketopiperazine (DKP) derived cyclic amidine structures widely exist in peptide natural products according to the genome mining result. The largely unknown bioactivity and mode of action are partially caused by the poor availability of the compounds via microbiological and chemical approaches. To tackle this challenge, in this work, we have developed the on-resin ring-closing amidine formation strategy to synthesize peptides containing N-terminal DKP derived cyclic amidine structure, in which the 6-exo-trig cyclization mediated by HgCl2 activation of thioamides was the key step. Leveraging from this new strategy, we finished the total syntheses of streptamidine and klebsazolicin. Meanwhile, eleven klebsazolicin analogues were synthesized for its structure–activity relationship study.
Introduction
Amidine is as an old chemical moiety with more than 100 years of history and has always been attractive to chemists for its unique chemical and physical properties.1 Amidines show strong basicity due to the effective delocalization of the positive charge in its protonated form, and can serve as both donors and acceptors engaged in the hydrogen bond interaction.2 The protonation state dependent hydrogen bond interaction of amidines with negatively charged species like carboxylate and bicarbonate makes it a useful building block in the stimulus-responsive material design.3 Amidine structures have been found in natural products like polycyclic alkaloids4 and bacterial polysaccharides. [5] Moreover, amidine is acknowledged as the bioisostere of an amide6 and has been applied in a series of small molecular drugs,7 agrochemicals,8 and drug candidates.9
Amidines are involved in both natural and artificial peptide backbones (Figure 1). In 2011, Boger et al. reengineered the cyclic peptide core of vancomycin via total synthesis to realize the dual D-Ala-D-Ala and D-Ala-D-Lac binding,10 which led to the recent development of maxamycin 1 with high potency against vancomycin-resistant pathogens.11 Bottromycin A2 2 is the first discovered cyclic amidine-containing peptide antibiotic, which shows good activity against Gram-positive pathogens including MRSA and VRE.12 The cyclic amidine structure was proposed based on NMR data in 1983,12b before the full structure elucidation and confirmation by total synthesis in 2009.13 The model cyclic peptide 3 was synthesized by Yudin et al. to probe the conformational change caused by the unique hydrogen bond interaction mode of the amidine.14 In recent years, genome mining technology was introduced to the discovery of cyclic amidine-containing peptide natural products, which led to the discovery of streptamidine 415 and klebsazolicin 5. [16] In 2017, during the mining of thiazole/oxazole-modified microcins (TOMMs), Severinov and Polikanov et al. unexpectedly discovered a novel peptide narrow-spectrum antibiotic klebsazolicin 5 containing the intriguing diketopiperazine (DKP) derived cyclic amidine structure at the N-terminus.16 In 2021, by mining YcaO-domain proteins and associated short peptides in Actinobacteria genome database, Truman et al. discovered streptamidine 4 as the second natural product containing DKP derived cyclic amidines.15 According to the Truman et al.’s result, the streptamidine-like biosynthetic gene clusters (BGCs) exist widely in nature, which suggests a beneficial role for the producing organisms. However, the totally unknown bioactivity is waiting for further investigation, which is hampered at least partially by the poor availability of those natural products via microbiological methods. The chemical synthetic approach toward DKP derived cyclic amidine will be of great importance to the exploration of biological mysteries of related natural products. Herein, we report the establishment of the biomimetic on-resin ring-closing amidine formation strategy towards DKP derived cyclic amidine structures, and its applications in the total syntheses of streptamidine 4 and klebsazolicin 5.
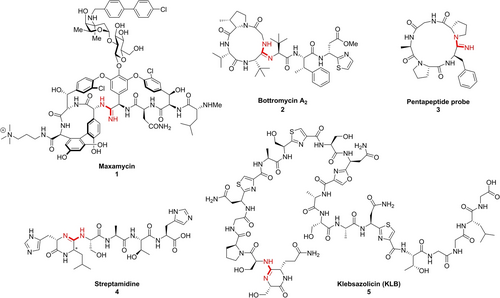
Natural and synthetic peptides containing amidine structures.
Results and Discussion
Several synthetic strategies for the introduction of cyclic amidine structures into peptide backbones have been developed in the past decade and applied to the synthesis of complex target molecules (Figure 2). In Sunazuka and Ōmura's total synthesis of bottromycin A2 2, the amidine bond was first generated intermolecularly from a linear dipeptide-like thioamide and N-terminal amine of another peptide (Strategy 1), followed by further chain elongation and peptide cyclization via amide coupling.13 Phthalimide protection of the N-terminus of the thioamide and using TBDPS protected ethanolamine as Gly surrogate were critical, indicating the high sensitivity of the Hg(OTf)2 mediated intermolecular amidine formation to competing nucleophiles. During the reengineering study of vancomycin, Boger et al. developed an alternative approach using the cyclic peptide derived thioamide as the amidine precursor (Strategy 2).10b In this case, large excess amount and high concentration of NH3 (as saturated solution in MeOH) was used to efficiently suppress other nucleophilic species, which led to the compatibility of the AgOAc mediated amidine formation with variable protecting group patterns. This strategy was also successfully applied to their recent development of maxamycin 1 and Yudin's synthesis of peptide 3.14 A new generation protocol using only 5 equiv. NH4OAc was recently achieved by VanVeller et al., through on-resin activation of thioamide to S-methyl thioimidate followed by aminolysis.17 The protocol showed robustness in the linear amidine containing peptides, while the effectiveness on cyclic peptide substrates is worth examination.
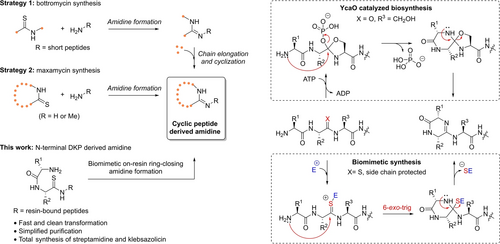
Reported synthetic strategies toward peptide derived cyclic amidine structures and biomimetic on-resin ring-closing amidine formation developed in this work.
In our initial synthetic study of peptides containing N-terminal DKP derived cyclic amidines, we met failure by adopting the above strategies. The relatively low reactivity of the peptide N-terminal amine and the impracticability of using the peptide in large excess amount hampered the intermolecular amidine formation. During the YcaO catalyzed amidine biosynthesis in the case of klebsazolicin, the amidine structure was directly generated on the full-length peptide via C=N bond formation between N-terminal amine and corresponding amide with spontaneous diketopiperazine ring closure.16b, 18 Inspired by this, we turned to design a biomimetic ring-closing amidine formation approach (Figure 2). Since the thioamide activation-based amidine synthesis is not compatible with competitive nucleophiles like hydroxyl (Ser/Thr), carboxylic acid (Asp/Glu) and carboxamide (Asn/Gln), full protection of side chain functional groups and C-terminal carboxylic acid is mandatory. Considering the generally poor solubility of protected peptides in organic solvents, we decided to develop an on-resin ring-closing amidine formation approach where the resin served as the C-terminal protection. We hypothesized that the resin bound thioamide containing peptide with protected side chains could serve as the precursor of amidines, and the kinetically highly favored 6-exo-trig cyclization could ensure the priority of the desired C=N bond formation under the thioamide activation over side reactions. Performing the amidine formation on resin-bound peptides will minimize the separation efforts and improve synthetic efficiency. Of course, many factors like resin swelling capacity of solvents and reagent solubility need consideration.
For process development, we chose the N-terminal pentapeptide derived from klebsazolicin 5 as the model structure. As shown in Figure 3a, starting from Fmoc-Gly-OH loaded 2-chlorotrityl (2-CTC) resin 6, Pro and Ser residues were installed sequentially via the standard Fmoc-SPPS. After releasing N-terminal amine, the thioamide formation was achieved by adopting Chatterjee's19 and Petersson's protocol20 using N-thioacyl benzotriazole reagent 8 as the thioamide donor (freshly generated by NaNO2/HOAc activation of thioamide derived from 4-nitro-o-phenylenediamine and Fmoc-Gln(Trt)-OH. [21] To suppress the racemization of reagent 8, 0.5 equiv. of DIPEA was used under the optimal conditions. After the thioamide formation, the last Ser residue was installed. Though the α-H of thioamide in 9 was less acidic than that in reagent 8, we observed significant epimerization at the thioamide α-C under standard Fmoc removal conditions (4-methylpiperidine : DMF 1 : 3 v/v, 20 min).22 Thus, a milder deprotection condition (4-methylpiperidine : DMF 1 : 7 v/v, 1 min ×2) was used in all the Fmoc removal steps after thioamide installation. With resin-bound peptide 10 in hand, we screened the conditions for on-resin ring-closing amidine formation (Figure 3b). To be compatible with on-resin reaction, a series of DMF soluble thiophilic metal salts were tested in combination with DIPEA. The reactions were monitored by UPLC analysis of the side chain protected peptide species after soft cleavage, because the globally deprotected amidine product was hardly retained by C18 column. CuCl2 gave <5% conversion after prolonged reaction, while AgOTf, AgOAc and Hg(OAc)2 gave low yields of amidine product 11 due to the formation of side products. To our delight, Hg(OTf)213 gave 45 % yield, which was improved to 55 % by using HgCl2 instead. Trace amount of water was found to be harmful to the reaction by generating formal hydrolysis product 12, and conducting the reaction under anhydrous conditions further improved the yield to 62 %. Though DIPEA was generally considered as a weak base and used in the thioamide formation and Ser1 installation steps, we observed significant epimerization during the amidine formation (up to 11 %). Since decreasing the amount of base led to partial cleavage of peptide from 2-CTC resin, we tested pyridine with weaker basicity. Under the optimized conditions, product 11 was formed in 86 % yield, with 8 % epimerization product epi-11 and negligible hydrolysis (Figure 3c). We also tested the thioamide activation by non-metal reagents like MeI,[17, 23] TfOMe24 and Meerwein's salt,25 but without positive results. Product 11 was purified by reversed phase preparative HPLC in 73 % yield and fully characterized by 1D and 2D NMR. The formation of cyclic amidine structure was unambiguously confirmed by HMBC. We also tested the scope of the on-resin ring-closing amidine formation by varying the N-terminal three amino acid residues of the model pentapeptide that were directly involved in amidine formation. As shown in Figure 3d, variation at all three positions gave comparable cyclization efficiency as illustrated by UPLC monitoring (Figure S130–S141), and products 13 a–13 f were isolated in a side chain protected form by reversed phase preparative HPLC in 29–60 % yields. This biomimetic chemical synthetic approach can overcome the Ser3 dependency of the YcaO catalyzed biosynthesis16b and allow the synthesis of products with higher structural diversity.
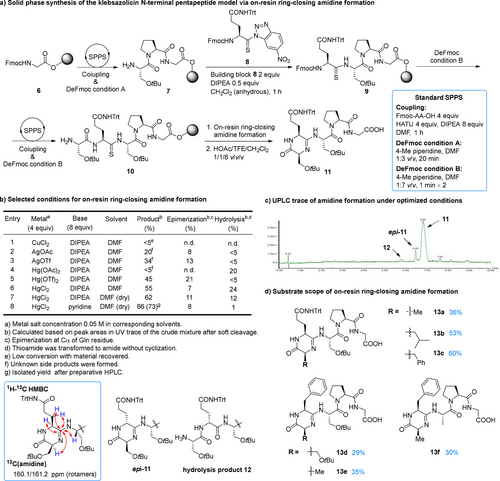
Exploration of the biomimetic on-resin cyclization via amidine formation. a) Solid phase synthesis of the klebsazolicin N-terminal pentapeptide model via on-resin ring-closing amidine formation. b) Selected conditions for on-resin ring-closing amidine formation. c) UPLC trace of amidine formation under optimized conditions. d) Substrate scope of the on-resin ring-closing amidine formation. DIPEA: diisopropylethylamine; DMF: N,N’-dimethylformamide; TFE: 2,2,2-trifluoroethanol; HATU: 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate.
Encouraged by the model studies, we moved further to the total synthesis of a newly identified cyclic amidine-containing natural product streptamidine 4. In the Truman's report,15 freshly purified streptamidine sample showed a single set of peaks in NMR, but the formation of more peaks was observed in the NMR medium in a time-dependent manner. From the amino acid analysis by the Marfey's method, the Leu2 was found to be a mixture of D- and L-forms, which was proposed to be caused by the epimerization. However, isolation and characterization of the epi-streptamidine was left blank.
In our synthetic endeavor (Figure 4a), starting from Fmoc-His(Trt)-OH loaded 2-CTC resin 14, three more amino acid residues were installed sequentially following standard Fmoc-SPPS protocols. The resin-bound tetrapeptide 15 was treated with 16 (prepared from L–Leu) and DIPEA in anhydrous CH2Cl2 to give rise to thioamide-containing peptide 17. After installation of the His1 residue using the standard coupling condition together with optimized Fmoc deprotection conditions (deFmoc condition B in Figure 3a), intermediate 18 was subjected to the on-resin ring-closing amidine formation (HgCl2 4 equiv, pyridine 8 equiv, anhydrous DMF). After global deprotection, streptamidine 4 a was isolated by reversed phase preparative HPLC in TFA salt form (40 % yield). We also synthesized the TFA salt of epi-streptamidine 4 b in 62 % yield by using ent-16 (prepared from D-Leu) during the thioamide formation step. As shown in Figure 4b, two epimers clearly showed different retention time in UPLC analysis. Both compounds were fully characterized by 1D and 2D NMR spectra as well as high-resolution MS. To further compare the synthetic streptamidine 4 a with the literature report, we changed the salt form into formic acid salt via repeated lyophilization from aqueous formic acid solution. During this process, we observed the formation of 4 b, though in tiny amounts. During the NMR analysis in DMSO-d6, the transformation of 4 a to 4 b became quite clear, and 4 a showed the same NMR spectra as the reported streptamidine. The equilibrium could be achieved after 48 h, where 4 b with more stable trans substitutions on the DKP ring became dominant. The same equilibrium could also be achieved from pure 4 b under the same conditions. The epimerization was not observed using TFA salt of 4 a or 4 b in both water and DMSO-d6. We proposed that this salt form dependent epimerization of 4 a could be attributed to the significantly higher pKa of formic acid in DMSO than in aqueous media. On the contrary, the weak basicity of trifluoroacetate anion in DMSO was not enough to cause epimerization.

Total synthesis of streptamidine and its epimer and elucidation of the undefined chirality. a) Total synthesis of streptamidine and epi-streptamidine. b) UPLC analysis of synthetic streptamidine and epi-streptamidine. c) Salt form dependent epimerization of streptamidine. TFA: trifluoroacetic acid; DMSO: dimethylsulfoxide; TIPS: triisopropylsilane; EDT: 1,2-ethanedithiol.
To further test the capability of the on-resin ring-closing amidine formation strategy, we next pursued the total synthesis of klebsazolicin (KLB) 5. This compound was discovered by Severinov and Polikanov et al. in 2017 during the genome mining based search of novel thiazole/oxazole-modified microcins (TOMMs).16a The unique structure of KLB comprised of multiple azole rings (3 thiazole rings at Asn6-Cys7, Ser9-Cys10, and Asn17-Cys18 sites; 1 oxazole ring at Asn12-Ser13 site) and N-terminal cyclic amidine, was unambiguously elucidated via extensive 2D NMR study (using 13C/15N enriched sample) and XRD analysis of the complex between KLB and 70S ribosome from T. thermophilus. KLB exerts its antibacterial activity through protein synthesis inhibition via binding to the peptide exit tunnel in 23S rRNA. The multiple hydrogen bond interactions and π–π stacking effects between the azole rings/amidine locating in Ser1-Ala14 region and rRNA nucleotides are involved to the complex stabilization, while the C-terminal tail Ser15-Gly23 is related to uptake mediated by transporters like OmpF and SbmA. The novel interaction mode of KLB with rRNA based on its curled conformation and uptake-related narrow antibacterial spectrum make it a good target for chemical synthesis and structural modification.
As illustrated in Figure 5a, starting from Fmoc-Gly-OH (Gly23) loaded 2-CTC resin 6, the 20 amino acids (Ser3 to Leu22) were installed following standard Fmoc-SPPS protocols to yield resin bound peptide 28. For the installation of thiazole structures, dipeptide thiazole building blocks 22 a and 22 b were prepared from the corresponding dipeptide derived thioamides 20 a and 20 b respectively, via Mitsunobu-type thiazoline formation26 and MnO2 oxidation27 (Figure 3b). During our synthesis of dipeptide oxazoline 24, we observed significant racemization at the Asn α-carbon (S : R 1.6–2.2 : 1) under all the tested conditions. Though not much precedent in literature,28 the incompatibility of the increased acidity of the α-proton in 24 and DBU was expectable. Since we failed to further improve the result by using other oxidation conditions like MnO2, NBS/BPO29, Cu(OAc)2/tert-butyl perbenzoate30, and DDQ31, we transformed 24 into separable epimers 26 a and 26 b by coupling with Fmoc-Ser(tBu)-OH after protecting group manipulations. The desired (S,S)-epimer 26 a was isolated and transformed to tripeptide oxazole building block 27 ready for SPPS by hydrogenolysis of benzyl ester. Treating 28 with 8 under anhydrous conditions gave rise to thioamide 29, which was subjected to the installation of the final Ser1 residue. In this case, the optimized Fmoc deprotection (condition B in Figure 3a) could suppress the epimerization of Gln2 α-carbon to less than 5 %, which could be removed after cyclization. In the ring-closing amidine formation step, addition of HgCl2 (4 equiv) and pyridine (8 equiv) to 30 successfully afforded the amidine product. However, formation of side products originating from the coordination of Hg2+ with thiazole rings in KLB was observed, which led to difficult purification and low yield. After screening, we found that adding thiophene (10 equiv) as competitive ligand of Hg2+ to the cyclization mixture successfully suppressed the Hg2+-KLB complex formation, while using thiazole (10 equiv) as additive gave slightly inferior result. After global deprotection, the KLB 5 was purified by reversed phase preparative HPLC in overall 10 % yield.
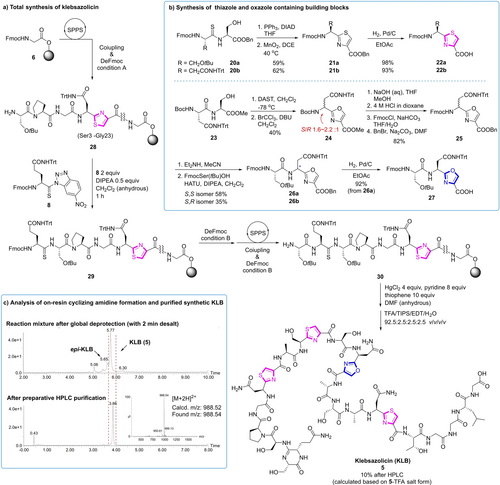
Total synthesis of klebsazolicin via on-resin ring-closing amidine formation. a) Total synthesis of klebsazolicin. b) Synthesis of thiazole and oxazole containing building blocks. c) Analysis of on-resin ring-closing amidine formation and purified synthetic klebsazolicin. THF: tetrahydrofuran; DCE: 1,2-dichloroethane; DIAD: diisopropyl azodicarboxylate; DAST: diethylaminosulfur trifluoride.
With the chemical synthetic approach of KLB established, we synthesized 11 KLB analogues 31 a–31 k, by changing the oxazole to thiazole (31 a) and replacing one or two of the oxazole/thiazole units to the corresponding linear AA-Ser form (31 b–31 k) (Figure 6). Since the N-terminal cyclic amidine was found to be important for its activity, it was kept intact during the structure–activity relationship (SAR) study. All the analogues were isolated by preparative HPLC in 7–24 % overall yields. The antibacterial activities of KLB 5 and analogues 31 a–31 k were tested on a series of clinically isolated pathogens, including both Gram-positive (B. cereus) and Gram-negative (E. coli, K. pneumoniae, and V. cholerae) ones showing resistance to colistin and/or carbapenem. As summarized in Table 1, in minimal M9+glucose medium, only KLB 5 and analogue 31 e with the thiazole D opened showed moderate growth inhibition activity (MIC 32 μg/mL to colistin resistant E. coli 5 strain, while all the compounds were inactive against E. coli CL4 and K. pneumoniae strains in our test. To our surprise, all the tested compounds showed good growth inhibition activity (MIC 0.125–0.5 μg/mL) against two V. cholerae strains (colistin sensitive/resistant), and most of the compounds showed good activity (MIC 0.125–0.5 μg/mL) against two B. cereus strains (carbapenem sensitive/resistant). As the positive control, the ribosome targeting antibiotic erythromycin showed better potency than KLB analogues against B. cereus and V. cholerae. According to our data, opening one or two azole rings could be well tolerated in the case of V. cholerae, indicating the curled conformation needed for rRNA binding was largely maintained. Totally lacking activity against all tested strains in nutrient-rich LB medium (Table S7) could be attributed to the disfavored uptake of the compounds by the pathogens where the nutrients compete for the transporters.
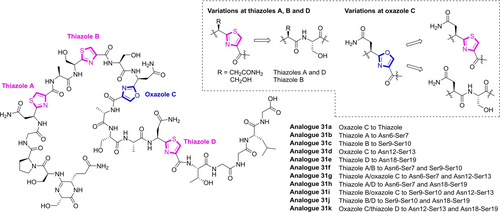
Structures of synthetic klebsazolicin analogues with modifications at thiazole and oxazole units.
Strains and phenotypes |
Ery |
5 |
31 a |
31 b |
31 c |
31 d |
31 e |
31 f |
31 g |
31 h |
31 i |
31 j |
31 k |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
E. coli |
5 (Col R) |
>128 |
32 |
>128 |
>128 |
>128 |
>128 |
32 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
CL4 (Car R) |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
|
K. pneumoniae |
Hvkp7 (Col/Car R) |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
FJ-8 (Car R) |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
>128 |
|
B. cereus |
BC177 (Car. S) |
0.0625 |
0.25 |
>128 |
32 |
0.125 |
0.125 |
128 |
0.25 |
0.125 |
0.125 |
0.125 |
0.125 |
0.125 |
BC107 (Car R) |
0.125 |
0.25 |
>128 |
>128 |
0.25 |
0.25 |
128 |
0.25 |
0.125 |
0.125 |
0.5 |
0.125 |
0.5 |
|
V. cholerae |
V1978 (Col S) |
0.0625 |
0.25 |
0.5 |
0.5 |
0.125 |
0.25 |
0.25 |
0.25 |
0.25 |
0.125 |
0.125 |
0.25 |
0.125 |
V1853 (Col R) |
0.0625 |
0.5 |
0.25 |
0.25 |
0.25 |
0.25 |
0.25 |
0.5 |
0.25 |
0.5 |
0.125 |
0.25 |
0.25 |
|
- [a] All the MIC data were obtained by treating bacteria cultured in M9+glucose medium with varying concentrations of synthetic klebsazolicin and its analogues. [b] Ery: erythromycin; Col: colistin; Car: carbapenem; S: sensitive; R: resistant.
Conclusions
In summary, we developed the on-resin ring-closing amidine formation strategy to synthesize peptides containing N-terminal DKP derived cyclic amidine structures. The 6-exo-trig cyclization via attack of free N-terminus to HgCl2 activated thioamides smoothly gave rise to the desired cyclic amidine structure, and the products in side chain protected or globally deprotected forms were obtained in good overall yields after single HPLC purification. Leveraging from this new strategy, we have finished the total syntheses of streptamidine and klebsazolicin and investigated the SAR of klebsazolicin by synthesizing 11 analogues. Since the wide existence of RiPP natural products containing DKP derived cyclic amidine structures according to the Truman's genome mining study,15 we believe the establishment of the efficient chemical synthetic strategy towards those natural products could accelerate the exploration of their biological activity and mode of action that are currently unknown.
Supporting Information
Supporting Information including experimental details and copies of UPLC-MS traces and NMR spectra (1D and 2D) can be found in the Supporting Information. The authors have cited additional references within the Supporting Information (Ref. [32]).
Acknowledgments
This work was supported by the Research Grants Council of Hong Kong (17306521, T11-104/22-R). X.L is the recipient of Research Grants Council-Senior Research Fellow Scheme (SPFS2324-7S01).
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




