Directional Bias in Molecular Photogearing Evidenced by LED-Coupled Chiral Cryo-HPLC
Abstract
Molecular gearing systems are technomimetic nanoscale analogues to complex geared machinery in the macroscopic world. They are defined as systems incorporating intermeshed movable parts which perform correlated rotational motions by mechanical engagement. Only recently, new methods to actively drive molecular gearing motions instead of relying on passive thermal activation have been developed. Further progress in this endeavor will pave the way for unidirectional molecular gearing devices with a distinct type of molecular machine awaiting its realization. Within this work an essential step towards this goal is achieved by evidencing directional biases for the light-induced rotations in our molecular photogear system. Using a custom-designed LED-coupled chiral cryo-HPLC setup for the in situ irradiation of enantiomeric analytes, an intrinsic selectivity for clockwise or counterclockwise rotations was elucidated experimentally. Significant directional biases in the photogearing processes and light-induced single bond rotations (SBRs) are observed for our photogear with directional preferences of up to 4.8 : 1. Harnessing these effects will allow to rationally design and construct a fully directional molecular gearing motor in the future.
Introduction
Molecular gears are a special type of molecular machinery performing correlated rotations of individual intermeshed molecular fragments.1 Research on this topic belongs to the earliest work on the synthetic miniaturization of macroscopic technology2 and has captured the imagination of chemists for a long time. A variety of molecular designs have been presented in this regard ranging from molecular brakes3 and speed-controllers4 to intermeshed molecular systems moving synchronously such as bevel5 and spur gears6 or surface-mounted gears and gear-trains.7 Recent systems also display stimuli-responsive behavior, e.g. light-controlled reversible engaging and disengaging of molecular gears by clutch mechanisms.8 However, most solution-based gearing motions result from thermal activation and active powering of the rotations was only achieved for surface-mounted systems by electrical stimulations.9 This restriction represents a serious problem as it prevents the application of molecular gears for redirection and speed-shifting of driven motions. Furthermore, unidirectional gearing at the molecular scale—akin to the inner workings of macroscopic motor engines or clockworks is only achievable in actively-powered systems. Directional rotations at the nanoscale per se are produced by molecular motors10 and great efforts are made to harness and transmit their motions to drive secondary ones.11 Gearing offers an ideal mechanism to achieve such motion translation but has yet to be brought under directional control. This is emphasized by a timely theoretical study presenting the feasibility of coupling molecular motor motions to spur-gear processes.12 Further, the possibility for intrinsically directional molecular gearing would represent an unprecedented integrated nanomachine, which merges the function of a motor with a gear.
We have recently presented the first light-powered molecular gearing motion, which we termed “photogearing” as it allows to drive a nanoscopic intermeshed bevel-gear with photon energy (Figure 1a).13 Photogear 1 is based on hemithioindigo (HTI) and consists of three molecular parts, a thioindigo, a triptycene, and an anisole fragment. The thioindigo and anisole fragments are connected by a photoisomerizable carbon-carbon double bond, which represents one central rotation axis. Rotation of the double bond can be evidenced directly via changes of its configuration from Z to E and vice versa, which represents a 180° rotation. The double bond is also connected to the triptycene fragment via a carbon-carbon single bond, which represents a second and directly adjacent rotation axis. This axis is angled by 120° to the double bond and rotation of the triptycene proceeds in 120° steps. Different options for rotations in this molecular setup exist, which can be distinguished by directly following the specific one-step isomer interconversions proceeding either thermally activated or light-induced. A sole double bond isomerization (DBI) or a sole single bond rotation (SBR) represent “slipping” motions in the terminology of gearing, as the thioindigo or the triptycene fragments only rotate around one axis (180° double bond or 120° single bond, respectively) without coupling of their motions. In contrast, the concomitant one-step rotation of both double- and single bond represents a true gearing motion, as both thioindigo and triptycene fragments rotate together in an intermeshed fashion. In such motion, the 180° rotation of the double bond is translated into a 120° rotation of the single bond while the rotation axis is also shifted by 120°. Indeed we found, that depending on the conditions, photogear 1 shows very distinct motions. While under thermal activation only gear slippage via uncorrelated SBR of the triptycene is observed, the input of light energy induces efficient photogearing with highly pronounced gearing fidelity for selected isomers. In our previous work we have thus established photogearing as a concept to actively power molecular gearing motions per se (Figure 1a). Although this is a first requirement for realizing actual unidirectional gearing, possible directionality of the motions in system 1 have not been investigated so far. This was owed to the inherent substantial difficulties to disentangle enantiomers and their motions in the thermally labile photogear. To solve this problem, we invented a specifically designed LED-coupled chiral cryo-HPLC setup and analytical method, allowing us to distinguish all possible rotation processes in photogear system 1. To the best of our knowledge such analytical setup is not available so far, despite earlier reports on cryo-HPLC applications. We believe that it will benefit anyone dealing with thermally labile photochemically active molecular systems and for that reason we describe it in detail in this dissemination as well. With this analytical method we deliver for the first time direct experimental evidence for intrinsic directional biases in a molecular gearing process (Figure 1b). Accompanied by a comprehensive, theoretical description and electronic circular dichroism (ECD) spectroscopic analysis, the photoconversion experiments revealed that the direction of photogearing is controlled by deliberate substitution at the triptycene moiety for photogear 1. Different to established mechanisms for molecular directionality control, the observed bias is heavily governed by electronic effects, which opens up entirely new opportunities for designing unidirectional nanomachnines in general. These findings further lay the foundation for an advanced future generation of molecular photogears, realizing sustained and autonomous energy-powered directional gearing at the molecular scale.
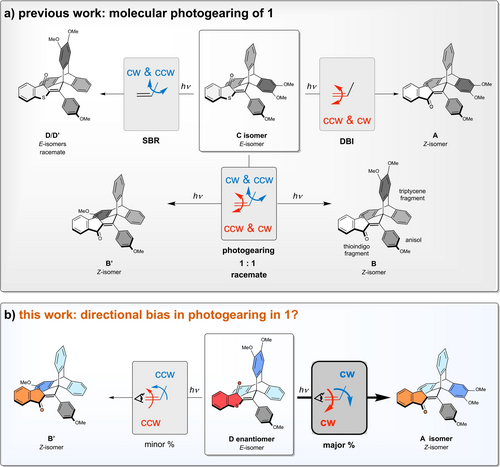
Photogearing and its directional biases in photogear 1. a) In the previous work SBR and DBI could be distinguished from the photogearing process (a simultaneous 180° DBI and 120° SBR rotation) and the latter experimentally evidenced. However, no directional biases were elucidated and only racemic mixtures were investigated. Note that only isomer D is shown as representative of the racemic D/D′ mixture obtained. b) In this work low-temperature separation of enantiomers, i.e. the separation and isolation of enantiomeric pairs B and B′ as well as D and D′, in situ irradiation of analytes, and the re-separation and quantification of the product mixture allowed uncovering significant directional biases in the photogearing motion.
Results and Discussion
Six isomeric states A, B, B′, C, D, and D′ are accessible for photogear 1, the first three possessing Z-configuration of the double bond and the latter three E-configuration (Figure 2 and Supporting Information, chapter 3). Isomers B and B′ as well as D and D′ are enantiomers and were not separately investigated before as the photogearing process itself is directly evidenced by the diastereomeric photoconversions between A and racemic D/D′ or C and racemic B/B′ (Figure 1a and Figure 2a). However, direct experimental evidence for the directionality of the photogearing process is possible for 1 and can be experimentally proven. For example, when starting from enantiopure state D, two directions of rotation are available for the photogearing process (Figure 1, bottom and Figure 2b). Photoconversion by clockwise 120° rotation of the triptycene fragment and implicit 180° clockwise rotation of the thioindigo fragment leads to isomer A. Photoconversion by 120° counterclockwise rotation of the triptycene in conjunction with the implicit 180° counterclockwise rotation of the thioindigo fragment transforms D to B′ instead. If irradiation of enantiopure D leads to an unequal population of the photoproduct states B′ and A, then a net directionality is evident for the process. At the same time, the directionality of the associated DBI is also predictable because of the necessity of concerted movements in a gearing process. Analogous inferences are made for photoconversions starting from enantiopure B, B′ and D′ but not for processes starting from the symmetric, nonchiral structures A or C. For symmetry reasons the latter starting states will inevitably lead to equal population of the two enantiomeric and degenerate states upon photogearing. Taken together, the pure enantiomers B, B′, D and D′ can be investigated for directional bias of their photogearing motions but a long-time operation of 1 under constant illumination will ultimately lead to racemization, as the non-chiral states A and C are inevitably populated. Although, a continuous directional movement can therefore not be achieved with 1, it allows to directly evidence directional photogearing for the subset of motions starting from four of its six isomeric states. Such evidence would not only establish directional photogearing to be existent in the first place, but would further deliver crucial information about the molecular design suitable for achieving such highly sophisticated motions. When comparing the behavior of the diastereomeric states B/B′ versus D/D′ more detailed insights can be obtained since different functional groups (sulfur in B/B′ and carbonyl in D/D′) that are intermeshed with the triptycene could alter the effectiveness and sense of directionality in photogearing.
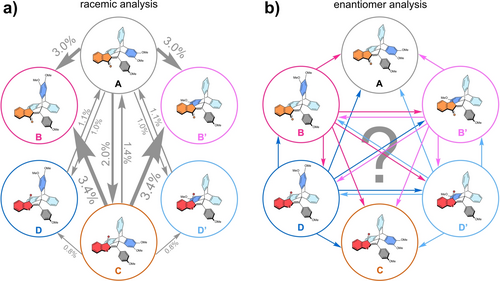
Quantification of the possible photoreactions of 1 as evidenced by individual isomer conversions. a) Racemic analysis by LED-coupled cryo NMR spectroscopy (CD2Cl2, −40 °C) with arrows and percentages in grey referring to photoquantum yields of previously established processes.13 b) Enantiomer analysis by LED-coupled chiral cryo-HPLC (pentane/ethyl acetate/i-PrOH or pentane/1,2-DCE/i-PrOH, −40 °C) with colored arrows referring to the specific transformation possibilities of hitherto hidden processes.
So far, possible directional biases of the photogearing rotations were not elucidated and also inter-enantiomer photoconversions between enantiomeric pairs B/B′ and D/D′, representing light-induced SBRs of the triptycene evaded observation. To follow these processes, simple separation of the enantiomers is not sufficient as photogear 1 undergoes rather fast isomerizations via thermal triptycene rotations at ambient temperatures. Therefore, we designed a LED-coupled chiral cryo-HPLC setup in order to reliably separate and isolate the enantiomers B/B′ and D/D′, store the isolated enantiopure solutions, irradiate the stored analytes, and finally separate the photoproducts after irradiation, all at low temperature (Figure 3).
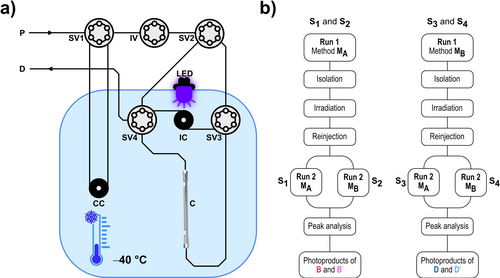
LED-coupled low-temperature chiral HPLC experiments to evidence directional photogearing. a) Schematic representation of the experimental setup (P: pumps, SV: switching valve, IC: irradiation coil, C: column, CC: mobile phase pre-cooling coil, D: detector). b) Simplified flow diagram of the chromatographic sequences S1–S4 used to obtain the photoproduct composition after an irradiation step. Repeating the sequences with varied irradiation durations allowed to record the photoconversion kinetics for each individual isomer A, B, B′, C, D, and D′.
The setup allowed us to perform the experiments at temperatures down to −40 °C (Figure 3a and Supporting Information, chapter 2.1). However, separation of all six isomers of photogear 1 in a single chromatographic run was not achieved on any chiral column tested. Therefore, using two separation methods MA and MB, we developed four chromatographic/irradiation sequences S1–S4 in order to capture the full performance of photogearing (Figure 3b and Supporting Information, chapter 2.2 and 7). Method MA allowed us to separate isomers A, B, B′, and C from racemic D/D′ and method MB separated isomers A, C, D, and D′ from racemic B/B′. While retaining constant irradiation intensities and temperatures for all sequences S1–S4, the photoconversions of enantiomers B, B′, D, and D′ were followed stepwise conducting two initial separations and two photoproduct separations for every irradiation duration. Performing the sequences with varied irradiation durations finally delivered the isomeric compositions after each irradiation step and hence the kinetic traces for the photoconversion of enantiopure samples. It should be noted that the solvent mixtures used for irradiation of B and B′ (method MA) was different to the one used for irradiation of D and D′ (method MA). In the former ethyl-acetate was used as medium polar component and in the latter 1,2-dichloroethane. The determined directional biases for each enantiomeric pair are thus specific to the conditions of irradiation and should be compared to each other with some caution across different solvent conditions.
For the determination of absolute configurations, the individual enantiomers B, B′, D, and D′ of photogear 1 were isolated at −40 °C using preparative cryo-HPLC and their ECD spectra were recorded in acetonitrile solution at −10 °C using a Peltier-cooled cuvette setup. Quantum chemical calculations at the M062-2X/cc-pVTZ level of theory allowed comparing experimental ECD spectra to theoretically obtained TD-DFT spectra and thus to elucidate the absolute configurations (Figure 4a,b and Supporting Information, chapter 3.2).
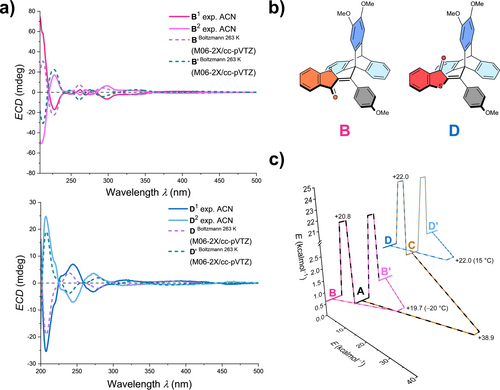
Determination of the absolute configuration of isomers B, B′, D, and D′ using cryo-ECD spectroscopy and TD-DFT calculations. a) Comparison of experimental ECD spectra of the enantiomers (acetonitrile, −10 °C, solid lines) with theoretical ECD spectra (M062-2X/cc-pVTZ level of theory, dashed lines). b) Schematic representation of isomers B and D used for theoretical description. c) Energy profile of photogear 1 (energy barriers without brackets refer to a common temperature of 30 °C, determined by VT NMR studies and Eyring plot analysis in a previous publication and energy barriers with the temperature in brackets were herein determined by cryo-HPLC).
We have previously determined that thermally induced motions in photogear 1 are restricted to only SBRs of the triptycene rotation, taking place within ca. 4 h at temperatures of −10 °C (B/B′ to A) to 15 °C (D/D′ to C). Only when heating to very high temperatures beyond 120 °C thermal DBI takes place as well. By preparative isolation and the subsequent annealing of enantiopure samples of B′ and D′, it was now also possible to elucidate the inter-enantiomer conversion kinetics for thermal SBRs interconverting B with B′ and D with D′ to complete the ground-state energy profile of 1 (Figure 4c, Supporting Information, chapter 6). As can be seen from the energy profile, thermal B/B′ to A and enantiomeric B to B′ conversions encounter very similar barriers giving rise to similarly fast kinetics. The same holds true for thermal D/D′ to C and enantiomeric D to D′ conversions.
The experimentally determined ground state energy profile was complemented by a theoretical description on the semiempirical GFN2-xTB and DFT B3LYP-GD3BJ/6-311G(d,p)/PCM(acetonitrile) level of theory (see Supporting Information chapter 4 for details). First, a semiempirical 2-dimensional scan of the double bond and the adjacent triptycene-bond coordinate was conducted, which confirmed selective thermal slipping of only the triptycene. The crucial minima and transition state points were then further optimized at the DFT B3LYP-GD3BJ/6-311G(d,p)/PCM(acetonitrile) level for quantitative comparison with the experimental values. Very good agreement between theory and experiment was found where the theoretical description reproduced very well the relative energies and order of minima as well as the transition state energies for triptycene rotations.
With the stereo configurations of all states and the energy profile completely elucidated, it became evident that irradiation experiments had to be conducted at −40 °C in order to completely exclude thermal SBRs from interfering with the photoreactions. The relative propensities pi/j for the photoconversions of enantiopure analyte solutions to all other isomers (induced by irradiation with light of 420 nm wavelength) were quantified as first-order rate constants of irreversible unimolecular reaction by the initial slope method. In the following we discuss normalized and enantiomer-averaged interconversion percentages %pi/j=pi/j ⋅ ∑pi/j−1 as fractions of the sum of all conversion propensities pi/j for each individual isomer (Figure 5, Supporting Information chapter 7).

Directional biases for photogearing motions and photoinduced SBRs of enantiomers B and B′ as well as D and D′ of photogear 1 evidenced by analysis of individual isomer conversions. Given values are normalized, enantiomer-averaged transformation percentages %pi/j of the sum of all photoreactions for a single isomer. Significant directional biases were found for both processes starting from isomers D and D′ and to lesser degree also for isomers B and B′. Opposite selectivity is seen for the photogearing biases between B/B′ and D/D′.
The conversion from B to A via a clockwise SBR represents a significant process with %pA/B=29 % interconversion percentage. The respective counterclockwise rotation leading from B to B′ is only slightly more pronounced with %pB/B′=33 %. Therefore, a directional bias for the SBR photoreaction was not observed for isomer B displaying a 1.1 : 1 ratio between counterclockwise and clockwise rotation of the triptycene fragment. Sole DBI transforming B to D is contributing with %pB/D=7 % conversion percentage. The photogearing processes of B are well distinguished in their proportions and also reach significant magnitudes compared to SBR and DBI transformations. Clockwise triptycene rotation correlated with clockwise thioindigo rotation leading from B to C is less likely with %pB/C=10 % compared to photogearing in opposite direction leading from B to D′ with %pB/D’=21 % (Figure 5). Therefore, a 2.1 : 1 directional bias in the photogearing of isomer B was evidenced along with an overall gearing fidelity of Fgear=%pgear ⋅ %pslip−1=0.45 for enantiomers B and B′. Concerning the directionality of the photogearing rotation, the motion for which the triptycene fragment is passing the thioindigo fragment via its methoxy-substituted blade is preferred (Supporting Information, Figure S55, S57). For E-isomeric D to D′, photogearing motions are significantly more pronounced than SBRs or DBIs with an overall gearing fidelity of Fgear=%pgear ⋅ %pslip−1=1.38. Irradiation of enantiopure D leads to a directional photogearing process populating isomer A as the most dominant pathway with %pD/A=48 % transformation percentage. Herein, the triptycene moiety rotates clockwise and passes the thioindigo fragment via its unsubstituted blade during the implicit clockwise rotation of the thioindigo fragment (Supporting Information, Figure S56, S57). The opposite counterclockwise photogearing motion to isomer B′ proceeds with %pD/B′=10 % transformation percentage, leading to a 4.8 : 1 directional bias (Figure 5). Light-powered SBRs also display a directional bias of 2.5 : 1, with a preference for clockwise rotation of the triptycene fragment converting enantiomer D to isomer C.
Interestingly, comparing Z-configured isomers B and B′ and E-configured isomers D and D′ both SBRs as well as photogearings display directional biases in opposite direction. Whereas for enantiomer B a counterclockwise triptycene rotation with implicated counterclockwise rotation of the thioindigo fragment in the photogearing process is favored, for isomer D a clockwise rotation of the triptycene with implicated clockwise rotation of the thioindigo fragment is observed as favored pathway. This strongly indicates an electronic origin of the directional bias in photogearing, because steric hinderance would consistently lead to rotations that avoid passing the thioindigo fragment with the methoxy-substituted triptycene blade. When starting from Z-isomeric structures with the electron deficient sulfur intermeshed with the triptycene, the electron rich methoxy-substituted triptycene blade rotates towards the sulfur atom during the gearing motion. On the contrary, in E-isomeric structures with the electron-rich carbonyl intermeshed, the opposite motion is preferred. Herein, contact of the carbonyl with the electron rich methoxy-substituted triptycene blade is avoided during photogearing. The dipole moments and electrostatic potentials of the ground states of photogear 1 are expected to be increased significantly for the excited state, similar to what was observed for previously studied HTI photoswitches, amplifying the electronic effects. This expectation is strongly supported by a theoretical natural transition orbital (NTO) analysis for isomers B and D (CAM-B3LYP/def2-SVP/CPCM(acetonitrile) level of theory, see Supporting Information chapter 5 for details). As can be seen from the NTOs the sulfur atom does indeed accumulate significant positive charge while the carbonyl group becomes much more negatively charged upon excitation.
It needs to be emphasized that the observed directional biases are not evidencing full directionality of the motions in photogear 1. During continued operation of 1 the enantiomeric states B/B′ and D/D′ will be equally populated eventually by unbiased transformations starting from isomers A and C, even if enantiomerically pure starting materials are employed initially. This gradual racemization leads to the cancellation of any directional motion performed by a particular enantiomer over time. Therefore, photogear 1 is not termed a molecular gearing motor, however, the observed directionality biases in photogear 1 represent highly valuable and guiding information for the construction of such an advanced machine. The electronic differences arising from non-symmetric substitution of the triptycene blades are evidently suitable to control the sense of directionality in photogearing processes. This directly leads to an informed design for an effective and overall fully directional photogear system, which would inherit a triptycene with three different blades as source of asymmetry. A suitable substitution pattern would be one neutral blade without substituents, one bearing electron donors, and one with electron acceptors. The resulting chiral photogear should show pronounced and persistent directionality control in its motion.
Conclusion
In conclusion the intrinsic directional bias of photogearing motions and light-induced single bond rotations are elucidated within molecular machine 1. Therefore, a novel LED-coupled chiral cryo-HPLC setup was developed allowing consistent isomer and enantiomer separation combined with in situ irradiation at low temperatures and hence the quantification of inter-isomer photoconversions. For light-induced single bond rotations opposite directional biases are observed for Z- and E-isomers of corresponding configuration, i.e. preference of counterclockwise rotation for B and D′ and preference of clockwise rotation for B′ and D. Also for the photogearing processes opposite directionality is observed with preference of counterclockwise rotation for B and D′ and preference of clockwise rotation for B′ and D. Directional biases are in more pronounced for the E-configured isomers D and D′ with a 4.8 : 1 bias for the transformation into isomer A. Thus, an intrinsic directionality of a molecular gearing process was evidenced for the first time. An electronic origin of the directional bias is proposed especially for rotation directions leading to sterically-congested transition states. The presented insights on biased rotations in molecular gearing systems pave the way towards the rational design of an effective light-powered molecular gearing motor. Our current efforts are targeted at the construction of such elaborate molecular machinery with continuous directional gearing performance.
Acknowledgments
H. Dube thanks the Deutsche Forschungsgemeinschaft (DFG) for an Emmy Noether fellowship (DU 1414/1-2, GEPRIS number 264598191). This project has also received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (PHOTOMECH, grant agreement No 101001794). Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




