Fabrication of Self-Expanding Metal–Organic Cages Using a Ring-Openable Ligand
Abstract
Metal–organic cages (MOCs), which are formed via coordination-driven assembly, are being extensively developed for various applications owing to the utility of their accessible molecular-sized cavity. While MOC structures are uniquely and precisely predetermined by the metal coordination number and ligand configuration, tailoring MOCs to further modulate the size, shape, and chemical environment of the cavities has become intensively studied for a more efficient and adaptive molecular binding. Herein, we report self-expanding MOCs that exhibit remarkable structural variations in cage size and flexibility while maintaining their topology. A cyclic ligand with an oligomeric chain tethering the two benzene rings of stilbene was designed and mixed with RhII ions to obtain the parent MOCs. These MOCs were successfully transformed into expanded MOCs via the selective cleavage of the double bond in stilbene. The expanded MOCs could effectively trap multidentate N-donor molecules in their enlarged cavity, in contrast to the original MOCs with a narrow cavity. As the direct synthesis of expanded MOCs is impractical because of the entropically disfavored structures, self-expansion using ring-openable ligands is a promising approach that allows precision engineering and the production of functional MOCs that would otherwise be inaccessible.
Metal–organic cages (MOCs) are discrete coordination compounds that can accommodate guest molecules in their cavity.1-4 Their structure is uniquely determined by the self-assembly of the metal nodes and multidentate organic ligands via reversible coordination bonds.5-7 The distinct ability of self-assembly to produce symmetric structures has led to the development of a wide range of MOCs with many functions, including molecular recognition, separation, delivery, and catalysis.8-14 In pursuit of host–guest chemistry based on MOCs, an obvious prerequisite for cage design is the size-tuning of the cavity for the efficient encapsulation of guest molecules. In this regard, the preparation of MOCs usually relies on aromatic linkers with a rigid conformation and configuration.5, 7 Although this approach affords robust cage structures with a defined cavity size for predictable molecular binding, tolerance and adaptability to diverse guest molecules are often lacking.
Recently, postsynthetic modification has emerged as a powerful method for rationally controlling the structures and properties of porous materials such as MOCs, metal–organic frameworks, and covalent organic frameworks.15-19 This approach has enabled the formation of functional derivatives with the preserved topology of the pristine materials, which enriches the properties of nanopores by using stimuli-responsive ligands, introducing new substituents and/or exchanging framework components.19-23 As for the tuning of pore size, the controlled removal of linkers or metal nodes can expand the initial cavity to a certain extent,24-27 although the most straightforward way is to use longer linkers to increase the distance between the metal nodes. However, the introduction of alternative linkers is often accompanied by a large structural deformation, causing serious structural damage to porous materials.28 Thus, a new postsynthetic modification strategy is required to achieve a drastic expansion of the cavity while maintaining the structural integrity of the original framework.
In this study, we propose a viable strategy for synthesizing self-expanding MOCs as a new class of molecular cages, in which the cavity size is significantly increased while retaining the cage topology (Figure 1). The key to accessing these unprecedented materials is the use of a ring-openable ligand with a flexible oligomeric chain tethering the two benzene rings of stilbene. The expansion of pristine MOCs is successfully triggered by the selective cleavage of the olefin bond in the stilbene moiety via ozonolysis on the basis of clip-off chemistry,29, 30 resulting in the formation of highly flexible cages that are inaccessible by direct synthesis using an analogous linear chain linker. This approach transforms otherwise non-porous MOCs into functional molecular cages with a high capacity for guest molecule accommodation, thus showing significant potential for engineering the structures and improving the properties of MOCs.
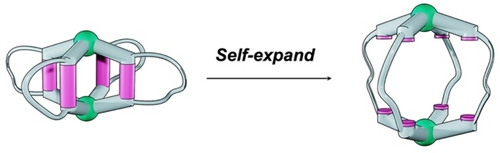
Illustration of the concept of self-expansion. This process involves the partial bond cleavage of the cyclic ligand.
To access self-expanding MOCs, we designed a dicarboxylate ligand (L1) with a rigid aromatic skeleton, in which the two benzene rings of the stilbene moiety are connected by a hexaethylene glycol (HEG) chain at the 2,2’ positions (Scheme 1 and Figures S1–S8). Among the many MOCs reported to date, we chose a RhII-based paddlewheel cage as an authentic structural motif because of its distinct structural stability and versatility.29, 31 An acidic form of the ligand (L1+2H) was mixed with Na2CO3 and rhodium(II) trifluoroacetate in N,N-dimethylacetamide (DMAc) at 100 °C for three days (Scheme 1 and Supporting Information). After reprecipitation from methanol, MOC 1 was obtained as a green powder in 84 % yield.
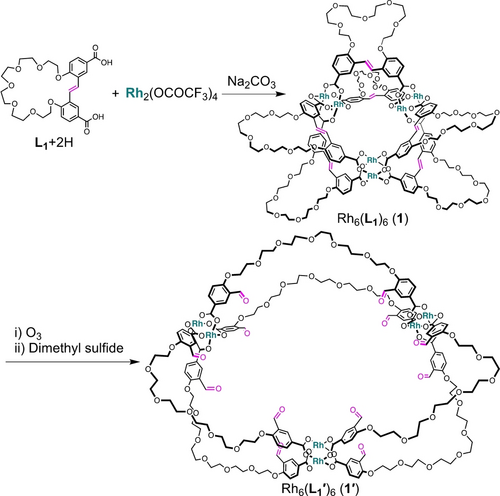
Synthetic Scheme for [Rh6(L1)6] (1) and its expanded structure (1′).
Formation of 1 as [Rh6(L1)6] was evidenced by matrix-assisted laser deposition/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). The MALDI-TOF-MS spectrum of 1 showed a sharp peak corresponding to the anticipated cage complex ([Rh6(L1)6+Na]+: expected=3907.6, found=3907.7) (Figure 2a). To characterize the structure of 1, we performed 1H nuclear magnetic resonance (NMR) spectroscopy in dimethylsulfoxide-d6 (DMSO-d6). The 1H NMR spectrum indicated a highly symmetric structure due to the geometric equivalency of the ligands (Figures 2b and S9). The diffusion coefficient (D), which is an intuitive parameter for probing molecular size, was measured by diffusion-ordered spectroscopy (DOSY). The decrease in the D value of the L1 unit from 1.3×10−10 m2 s−1 (logD=−9.9) to 6.3×10−11 m2 s−1 (logD=−10.2) indicate an increase in molecular size during the reaction, supporting the successful complexation between L1 and RhII ions (Figure S10). The structure of 1 was also analyzed by single-crystal X-ray diffraction.32 Single crystals of 1 were prepared by slow vapor diffusion of diisopropyl ether into an N,N-dimethylformamide (DMF) solution of 1 at ambient temperature. As shown in Figure 3a, the crystallographic structure of 1 is a cage molecule consisting of three dirhodium paddlewheel units linked by six L1 ligands (Figure S11, Table S1).
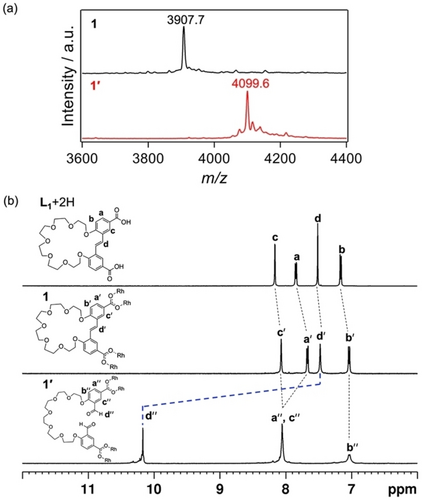
(a) MALDI-TOF-MS analysis of 1 and 1′. The mass peaks corresponding to [Rh6(L1)6+Na]+ ([1+Na]+) (expected=3907.6, found=3907.7) and [Rh6(L1′)6+Na]+ ([1′+Na]+) (expected=4099.7, found=4099.6) are highlighted. (b) 1H NMR spectra (aromatic region) of the acidic form of L1, 1, and 1′ (500 MHz, DMSO-d6, 298 K).
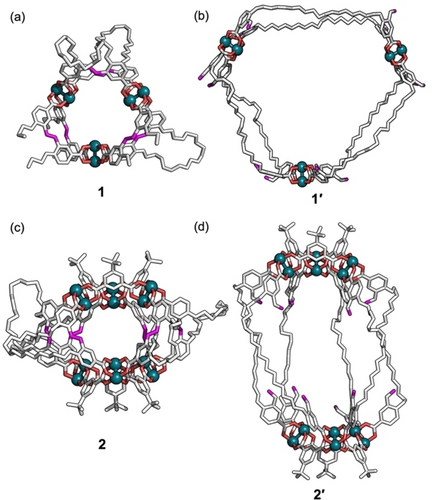
(a) X-ray crystallographic structure of 1 and molecular modeling structures of (b) 1′, (c) 2, and (d) 2′. For the structural analysis of 1, positions of HEG chains were partly undeterminable due to the disordered structure. Hydrogen atoms, solvent, and disorder are omitted for clarity. RhII ions, O atoms coordinated to RhII ions, and C atoms in double bonds are shown in green, red, and magenta, respectively.
The self-expansion of 1 proceeded via the selective cleavage of the double bonds in L1, producing a highly expanded MOC (1′) composed of a long flexible dicarboxylate ligand with aldehyde moieties (L1′: L1+2O). In this process, ozone was bubbled through the solution of 1 in mixed DMF/dichloromethane for 30 min at −78 °C, followed by treatment with dimethyl sulfide overnight at room temperature (Scheme 1).26 MALDI-TOF-MS corroborated the formation of 1′ ([Rh6(L1′)6+Na]+: expected=4099.7, found=4099.6) (Figure 2a). The reasonable mass increase from 1 to 1′ is due to the conversion of one C=C double bond to two aldehyde groups. Moreover, the quantitative dissociation of all double bonds in 1, together with the appearance of aldehyde groups, was confirmed by Fourier-transform infrared (FT-IR) and 1H NMR spectroscopies (Figures 2b, S12, and S13). During the self-expansion of 1, the dirhodium paddlewheel structure remained intact. This was confirmed by the ultraviolet-visible (UV/Vis) absorption spectrum, which showed no obvious change in the p*→s* transition of the RhII−RhII bond (Figure S14).33, 34 All these results indicate that the original cage 1 was successfully converted into 1′ with the structure expanded but retaining its structural integrity. Molecular modeling of 1′ indicated a structure with a considerably large and effective void space inside the MOC, in which the distance between two paddlewheel units in 1′ (2.5 nm) was more than three times as long as that in the original cage (0.7 nm) (Figure 3).
The preparation of 1′ or similar MOCs with long flexible linkers via direct synthesis using the corresponding ingredients (Figure S15 and S16) is impractical because of the entropically disfavored structures. In fact, mixing RhII ions with a terminally carboxylate-functionalized HEG linker as an L1′ analog did not produce the target MOC but afforded insoluble coordination networks (Figure S17). Therefore, highly expanded flexible MOCs are only accessible via a self-expansion approach using designed linkers.
The versatility of this self-expansion strategy was demonstrated by preparing other cages using multiple ligands. In addition to L1, tert-butyl isophthalate (L2) was mixed with rhodium(II) acetate in DMAc at 100 °C for three days, providing a cage complex with a composition of [Rh12(L1)6(L2)6] (2). Although 2 with a deficit of L1 was slightly detectable, 2 with a spherical cage geometry was predominant, as strongly suggested by molecular modeling, 1H NMR spectroscopy, and MALDI-TOF-MS (Figures 3c, S18 and S19).29, 35 Ozonolysis of 2 produced an expanded MOC (2′) (Figure 3d). FT-IR, UV/Vis absorption, NMR, and MALDI-TOF-MS measurements of 2′ suggested that the self-expansion of 2 proceeded in the same manner as that of MOC 1 (Figures S13, S14, S19, and S20).
The guest inclusion ability of these MOCs was examined using multidentate N-donor compounds such as 1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene (timb; N−N distance=1.1 nm) and 2,4,6-(tri-4-pyridinyl)-1,3,5-triazine (tpt; N−N distance=1.0 nm). First, we attempted to encapsulate them in the cavity of the parent MOC 1; however, the DMF solution of 1 became turbid with a drastic color change after the addition of either timb or tpt (Figures 4a and S21). This was because the cavity of 1 (RhII−RhII distance=0.7 nm) was too small to capture these molecules (Figure 3a). The MOC 1 did not allow guest inclusion inside its cavity, probably resulting in the formation of network structures through the coordination of the linkers at the exterior RhII sites (Figure 4a, bottom-right). In contrast, mixing these N-donor molecules and the expanded MOC 1′ in DMF afforded a clear solution (Figures 4a and S14). An obvious blue shift of the absorption band of the dirhodium paddlewheel bond was observed upon the addition of timb (Figure S14), suggesting effective binding to 1′ via coordination interactions (Figure 4a, bottom-left).34, 36 The 1H NMR spectrum of the complex (1′+timb) in DMSO-d6 showed the downfield shift of the peaks of timb, confirming its coordination to the dirhodium paddlewheel units (Figures 4b and S22). Quantitative analysis of 1′+timb by 1H NMR spectroscopy revealed the formation of a 1 : 1 host–guest complex (Figure S22). In addition, 1H DOSY NMR measurements showed that the mobility of timb was identical to that of 1′ owing to the encapsulation of the guest molecule in 1′ (Figure 4c).
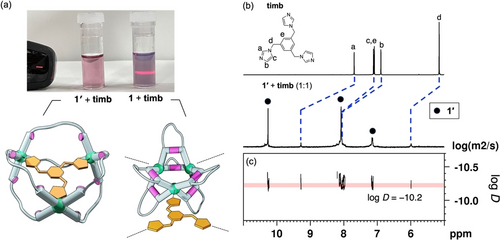
(a) Photos of DMF solutions (top) and the illustrations (bottom) of 1′+timb and 1+timb (timb: 1,3,5-tris[(1H-imidazol-1-yl)methyl]benzene). The expanded MOC (1′) gave a clear solution because of the effective entrapment of timb in its cavity. In contrast, the pristine MOC (1) gave a colloidal dispersion with Tyndall effect upon the addition of timb probably because of the formation of coordination networks. (b) 1H NMR spectra of timb and 1′+timb (500 MHz, DMSO-d6, 298 K). (c) 1H DOSY NMR analysis of 1′+timb (500 MHz, DMSO-d6, 298 K).
The same trends were observed in the UV/Vis and NMR analyses using tpt as the guest molecule, indicating the formation of a host–guest complex (1′+tpt) (Figures S14, S23–S25). Owing to the structural expansion of its skeleton, 1′ could capture guest molecules in its cavity. A single crystal of 1′+tpt was successfully obtained by slow diffusion of diethyl ether in the DMF solution of 1′+tpt. Single-crystal X-ray diffraction32 of 1′+tpt clearly revealed the formation of a 1 : 1 complex between 1′ and tpt, in which the guest tpt was efficiently trapped by 1′ via chelation at the interior dirhodium paddlewheel sites (Figures 5 and S26, Table S1). Remarkably, the expanded host adaptively captured the guest molecule in an induced-fit manner with the aid of its highly flexible structure with long HEG chain ligands.
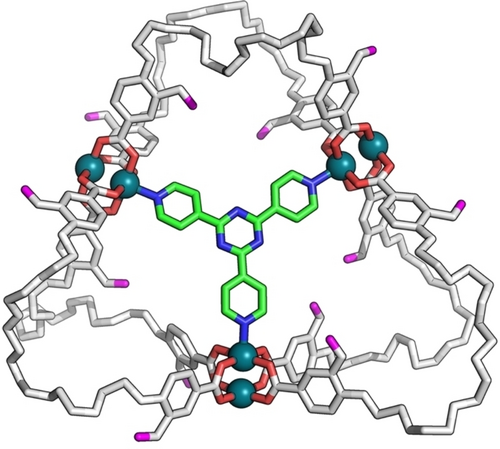
X-ray crystallographic structure of 1′+tpt (tpt: 2,4,6-(tri-4-pyridinyl)-1,3,5-triazine). Hydrogen atoms, solvent, and disorder are omitted for clarity. The C and N atoms of tpt are shown in green and blue, respectively.
In conclusion, we successfully synthesized self-expanding MOCs using a ring-openable ligand composed of a cleavable C=C bond and flexible oligomeric chain. The expansion of the MOCs without loss of structural integrity was enabled by the selective cleavage of the olefin bond, affording highly flexible cages capable of guest molecule capture. Because a variety of oligomers and polymers with different chain lengths can be employed for this method, it is possible, in principle, to design multicyclic polymeric cages with well-defined geometries. The method can not only be applied in the fabrication of new molecular cages for molecular recognition, but also contribute to the development of topological polymers with exceptional functions.
Supporting Information
The authors have cited additional references within the Supporting Information.37-41
Acknowledgments
This work was supported by “Advanced Research Infrastructure for Materials and Nanotechnology in Japan (ARIM)” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Grant Number JPMXP1223UT0127. A. N. acknowledges the 1st Kao Crescent Award, R4 Kumagai Research Grant, and KAKENHI Grant-in-Aid for Early-Career Scientists (JP23 K13789) from JSPS. T. U. is grateful for the Data Creation and Utilization-Type Material Research and Development Project (JPMXP1122714694) of the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




