Hierarchical Self-Assembly of Multidimensional Functional Materials from Sequence-Defined Peptoids
Abstract
Hierarchical self-assembly represents a powerful strategy for the fabrication of functional materials across various length scales. However, achieving precise formation of functional hierarchical assemblies remains a significant challenge and requires a profound understanding of molecular assembly interactions. In this study, we present a molecular-level understanding of the hierarchical assembly of sequence-defined peptoids into multidimensional functional materials, including twisted nanotube bundles serving as a highly efficient artificial light harvesting system. By employing synchrotron-based powder X-ray diffraction and analyzing single crystal structures of model compounds, we elucidated the molecular packing and mechanisms underlying the assembly of peptoids into multidimensional nanostructures. Our findings demonstrate that incorporating aromatic functional groups, such as tetraphenyl ethylene (TPE), at the termini of assembling peptoid sequences promotes the formation of twisted bundles of nanotubes and nanosheets, thus enabling the creation of a highly efficient artificial light harvesting system. This research exemplifies the potential of leveraging sequence-defined synthetic polymers to translate microscopic molecular structures into macroscopic assemblies. It holds promise for the development of functional materials with precisely controlled hierarchical structures and designed functions.
Introduction
Nature is a great masterpiece that creates vast and intricate living organisms using only around 100 kinds of elements.1 In this remarkable process, sequence-defined biomacromolecules, particularly polypeptides and nucleic acids, play an indispensable role in the assembly of hierarchical functional materials that exhibit remarkable functions,2 such as natural protein assemblies for light harvesting systems.3 Inspired by nature, scientists have developed various sequence-defined synthetic polymers to mimic the structures and functions of these natural biomacromolecules, aiming to create hierarchical materials that are highly robust while include massive amount of information.1a, 2b, 4 In contrast to the challenges associated with achieving precise control over molecular organization and interactions in proteins and peptides for the development of multidimensional hierarchical materials, utilizing sequence-defined synthetic polymers offers a solution with reduced complexity and improved control over molecular interactions. This approach holds immense significance in advancing the field of hierarchical materials.
Peptoids, as one of the most advanced sequence-defined synthetic polymers, have shown unique advantages for the development of hierarchical materials due to the structural similarity to peptides and proteins but lack of backbone hydrogen bond donors.1a, 2b, 5 By employing iterative solid-phase synthesis, the molecular sequence of peptoids can be precisely controlled, resulting in products that are both pure and well-defined. Additionally, the molecular interactions among peptoids can be predictably controlled through side-chain modifications.2b, 5b As a result, peptoids have been frequently used as tunable building blocks for constructing one-dimensional (1D) and two-dimensional (2D) hierarchical assemblies.2b, 5b, 6 Despite these efforts, it remains a challenge to develop long-range ordered, three-dimensional (3D) hierarchical materials. Achieving and controlling the order in such assembled materials demands a deep understanding of the principles that govern peptoid assembly interactions and the ability to manipulate these interactions precisely. In this study, by manipulating peptoid assembly interactions through side-chain variations and attachment of tetraphenylethylene (TPE), we successfully developed helical 3D nanostructures, including pH-responsive nanotube bundles exhibiting a highly efficient Förster resonance energy transfer. This work not only highlights the importance of manipulating peptoid assembly interactions in directing the formation of 3D hierarchical materials, but also provides insights for the rational design of multidimensional hierarchical materials with controlled structures and desired functions from sequence-defined synthetic polymers.
Results and Discussion
Design of the Peptoids Sequences
Benefitting from the well-developed ‘sub-monomer’ solid-phase synthesis method,6f, 7 peptoids with specific sequences can be easily synthesized in a high yield. Inspired by our previous work,2b, 5b, 6b-6d, 8 the peptoid sequences we designed here are all amphiphilic molecules, which incorporate both nonpolar and polar sub-blocks. The design principles can be classified into four types: I) Different residues are introduced as the polar or nonpolar domains. For example, either N-[(4-bromophenyl)methyl] glycines (Nbrpm) or N-[(4-bromophenyl)ethyl] glycines (Nbrpe) is introduced as the nonpolar domain, and N-(2-carboxyethyl)glycine (Nce) or N-(2-aminoethyl)glycine (Nae) is used as the polar domain; II) For the tri-block peptoid, the position of the polar and non-polar domains is changed to make bola-amphiphiles or di-block amphiphiles; III) The length of the nonpolar domain is adjusted by varying the number of residues; IV) Functional groups are covalently attached to the assembling peptoids, endowing these peptoids with unique optical properties and controllable self-assembly outcomes. Aromatic tetraphenylethylene (TPE) and its derivative (TPEPy) were introduced here as the functional group because they possess aggregation induced emission (AIE) property with the formation of potential π–π interactions,9 thus endowing the self-assembled peptoid materials with bright fluorescence emission. TPEPy was synthesized by modifying the TPE moiety with two methoxy group and one pyridine group, exhibiting a red-shifted absorption spectrum.9 Because the absorption spectrum of TPEPy overlaps with the emission spectrum of TPE,9 co-assembly of TPE- and TPEPy-attached peptoids could provide a new platform to construct highly efficient artificial light harvesting systems. In addition, the non-covalent interactions between these aromatic TPE and TPEPy groups are expected to tune the peptoid assembly interactions and regulate the self-assembly outcomes.10 Following these principles, nine peptoid sequences are designed and synthesized (PEP-1–PEP-9, Figure 1a). The purified peptoids are confirmed by ultra-performance liquid chromatography (UPLC) coupled with mass spectroscopy (MS) (UPLC-MS) (Figures S3–S11).
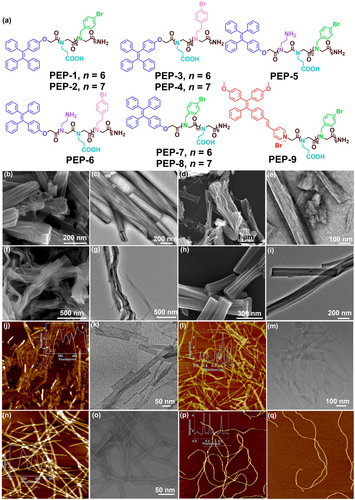
(a) Chemical structures of peptoid sequences PEP-1–PEP-9. SEM images of peptoid assemblies of (b) PEP-1; (d) PEP-3; (f) PEP-4; (h) PEP-9; and TEM images of peptoid assemblies of (c) PEP-1; (e) PEP-3; (g) PEP-4; (i) PEP-9. AFM images of peptoid assemblies of (j) PEP-5; (l) PEP-6; (n) PEP-7; (p, q) PEP-8. TEM images of peptoid assemblies of (k) PEP-5; (m) PEP-6; (o) PEP-7.
Assembly of Peptoids into Hierarchical Materials with Various Dimensions
Peptoid assemblies were obtained using an evaporation-induced crystallization approach as previously reported.6b, 6c, 6f Specifically, peptoids were first dissolved in a mixture solution of water (H2O) and acetonitrile (CH3CN) to obtain a clear solution, followed by a controlled slow evaporation at 4 °C. As a result, either gels or precipitates of peptoid assemblies were obtained after several days (see Supplementary Methods for details). Atomic force microscopy (AFM), transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were employed to characterize peptoid assembly morphologies.
Our characterization results show that the morphology of peptoid assemblies can be classified into two distinct types: 3D bundle structures assembled from peptoid sequences PEP-1, PEP-2, PEP-3, PEP-4, and PEP-9. As shown in the SEM and TEM images, PEP-1 self-assembled into nanotube bundles (Figure 1b and 1c) with the diameter of 80–150 nm and the length reaching micrometers. Similar nanotube bundles were formed using PEP-2 with seven Nbrpm residues as the hydrophobic domain (Figure S13). In contract, PEP-3 with six Nbrpe residues as the hydrophobic domain formed bundles of nanosheets with an average width of around 100 nm (Figure 1d and 1e). Such similar nanosheet bundles were also observed from the assembly of PEP-4 with seven Nbrpe residues (Figure 1f and 1g). TEM and SEM images showed that PEP-9 with TPEPy as the functional group assembled into nanotube bundles similar to those assembled from PEP-1 (Figure 1h and 1i).
Another type of morphology is the discrete tube or sheet nanostructure assembled from peptoids PEP-5, PEP-6, PEP-7 and PEP-8. As shown in the AFM and TEM images, PEP-5 self-assembled into nanotubes with an average diameter of 8 nm (Figure 1j and 1k). PEP-6 formed narrow nanosheets with the height of around 4 nm (Figure 1l and 1 m). PEP-7 formed well-defined nanotubes with an average diameter of 6 nm (Figure 1n and 1o). PEP-8 formed long and entangled nanotubes with the height of about 5 nm (Figure 1p and 1q).
Mechanistic Study of Peptoid Nanotube Bundle Formation
Among all peptoid assemblies we obtained, it is interesting to point out that the diblock peptoids with Nae (PEP-5/PEP-6) or Nce (PEP-7/PEP-8) as hydrophilic residues assemble into discrete tube or sheet nanostructures. In contrast, bola-amphiphilic peptoids with Nce residues as the hydrophilic domain (PEP-1–PEP-4 and PEP-9) form 3D nanotube or nanosheet bundles. To explore the impact of chemical structures on peptoid assembly outcomes, synchrotron-based powder X-ray diffraction (PXRD) was employed to gain further insights into the molecular structure of peptoid assemblies. As shown in Figure 2a, characteristic peaks corresponding to the distance between two peptoid backbones with two nonpolar residues facing each other are observed for specific peptoid assemblies (1.70 nm for PEP-5 and PEP-7, 1.60 nm for PEP-1 and PEP-9, 1.80 nm for PEP-3).6b, 6c, 6f PEP-3 with Nbrpe as the nonpolar domain has the peak at around 1.80 nm, which is in accordance with the typical sheet-forming sequence. While peptoids with Nbrpm as the nonpolar domain typically show the characteristic peak at around 1.67 nm for their assemblies, peptoid assemblies of PEP-5 and of PEP-7 both have the related peak at around 1.70 nm, and assemblies of PEP-1 and of PEP-9 show a peak of 1.60 nm. Such increased d-space of PEP-5 and PEP-7 assemblies could be due to the charge repulsion caused by Nae group of PEP-5 and the steric hindrance of TPE group in the hydrophobic domain of PEP-7. For assemblies of PEP-1 and of PEP-9, interactions between TPE or TPEPy groups could facilitate a closer packing of peptoid backbones, thus leading to the decreased d-space. Peaks at around 4.6 Å ascribed to the spacing between two adjacent peptoids and π-stacking related peaks (4.3, 4.0, and 3.8 Å) are also observed in the assemblies of PEP-1, PEP-3, PEP-5, PEP-7 and PEP-9. The peak of 3.0 Å is corresponding to the distance between two adjacent side chains of a cis-conformation peptoid.11 It should be noted that unlike assemblies of other peptoids, assembled PEP-7 materials show a broadened peak at around 4.5 Å due to the influence of TPE groups on the alignment of Nbrpm domains, lowering the crystallinity (Figure 2a and 2h). Overall, from the XRD peaks we obtained here and compared with those from previously reported nanosheets and nanotubes,6b, 6c, 6f, 8a, 12 we conclude that these amphiphilic peptoids are stacked with each other via a typical bilayered structure, in which the hydrophobic domains are closely stacked and the hydrophilic residues are distributed on the surface.
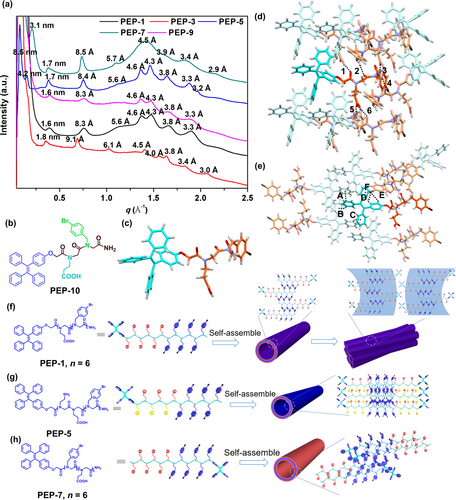
(a) XRD spectra of peptoid assemblies. The values above each peak are calculated according to the formula of d=2π/q. (b) Chemical structure of PEP-10. (c) Single crystal structure of PEP-10. (d) One highlighted PEP-10 molecule and the surrounding molecules that establish interactions with its backbone. Black dashed lines indicate the O−H⋅⋅⋅O interactions (1, 6) and N−H⋅⋅⋅O interactions (2, 3, 4 and 5). (e) One highlighted PEP-10 molecule and the surrounding molecules that establish interactions with its TPE group. Black dashed lines indicate the π–π stacking interactions (A), C−H⋅⋅⋅π interactions (B and C), C−H⋅⋅⋅Br interactions (D and E) and C−Br⋅⋅⋅π interactions (F). Proposed model showing the assembly of specific peptoids and the molecular packings within specific peptoid assemblies of (f) PEP-1, (g) PEP-5, and (h) PEP-7.
To further investigate the influence of TPE group on the peptoid packing, a short model compound peptoid PEP-10 containing one Nbrpm, one Nce and one TPE was synthesized (Figure 2b). By slowly evaporating the solution of PEP-10, we were able to successfully obtain single crystals suitable for X-ray crystallography and reveal the atomic level molecular interactions between peptoids (Figure 2c). Single crystal structure shows that PEP-10 molecules are packed closely with each other, and solvent molecules are not involved with peptoid interactions. It should be noted that peptoids backbones are stacked together through the formation of six hydrogen bonding interactions (1–6, Figure 2d). The hydrogen bond donors are from the carboxylic acid side chain and the terminal amide group, and backbone oxygen atoms and the carboxylic acid side chain afford the hydrogen bond acceptors. Those hydrogen bond lengths of 1–6 are calculated to be 1.88, 2.09, 2.02, 2.09, 2.02 and 1.88 Å, respectively. All distances are shorter than the sum (2.7 Å) of van der Waals radii of the hydrogen and oxygen or nitrogen atoms, indicating strong hydrogen bonding interactions.13 While TPE moieties are not involved in these hydrogen bonding interactions, they form π–π stacking, C−H⋅⋅⋅π, C−H⋅⋅⋅Br and C−Br⋅⋅⋅π interactions to stabilize peptoid assembly (Figure 2e, the parameters of these non-covalent bonds can be found in Supporting Information, Section 4).9a, 13, 14 The related centroid-centroid distance and dihedral angle of the π–π stacking interaction (A) are measured to be 4.76 Å and 0°. The relatively large centroid-centroid distance is attributed to a limited overlap of two benzene rings. Two C−H⋅⋅⋅π interactions (B and C) are observed among benzene rings of the TPE group (Figure 2e). The C−H⋅⋅⋅π distances and angles are measured to be 2.34 Å and 149.56°, respectively. The presence of extensive interactions among TPE groups suggests that TPE and its derivative TPEPy contribute significantly to the formation of 3D bundles of nanotubes or nanosheets during peptoid assembly. In contrast, an analogous peptoid with non-aromatic terminal group (PEP-al with hexyl as the end group, Figure S12) fails to induce the formation of these distinctive bundles (Figure S14).
Based on the PXRD and single crystal structure results, a probable mechanism is proposed to explain the different outcomes of peptoid assembly. For bola-amphiphilic PEP-1–PEP-4 and PEP-9, they first assemble into individual tubes with a bilayer-like packing of peptoids and TPE groups locating on the tube surface. As revealed in the crystal structure of PEP-10, TPE groups tend to interact with each other through extensive interactions including π–π stacking, thus facilitating the interactions among nanotubes to form 3D tube bundles (Figure 2f, using PEP-1 assembly as an example). For PEP-5 and PEP-6, the protonation of Nae groups within the hydrophilic domain results in the charge repulsion among peptoids, hindering the formation of nanotubes or nanosheets bundles (Figure 2g, using PEP-5 assembly as the representative). For PEP-7 and PEP-8, because the TPE groups are located at the end of hydrophobic domain, they form a bilayer-like packing of peptoids with TPE groups locating inside the hydrophobic domains of peptoid nanotubes. Thus, TPE groups on PEP-7 and PEP-8 impact the crystallinity of assembled peptoid nanotubes, but not contribute to the interactions among tubes, leading to the formation of discrete nanotube structures (Figure 2h, using PEP-7 assembly as an example).
To further understand the mechanism of peptoid assembly into bundles and bundle formation pathways, we used SEM to capture the PEP-1 assembly at different stages. As shown in Figure 3, after five hours, peptoids assembled into nanospheres with the diameter ranging from ~50 nm to ~400 nm (Figure 3a). These spheres were then gradually split into relatively small spheres of around 50 nm diameter, and later fused together to form a “beads-on-a-string” like morphology (Figure 3b). After 15 h, nanotubes emerged from these nanospheres (Figure 3c). At this stage, individual nanotubes formed first, and then intertwined and assembled into tube bundles. Gradually, the inner region of nanospheres also transformed into nanotubes and bloomed into a bundle of nanotubes (Figure 3d). These nanotubes further entangled and twisted to form nanotube bundles (Figure 3e). With time passing, an increasing number of tube bundles formed (Figure 3f). After around 72 h, almost all PEP-1 assemblies have well-defined tube bundle structures (Figure 3g). In brief, compared with the previously reported assembly pathway of discrete peptoid nanotube, which is through a “rolling-up and closure of nanosheet” process,6b PEP-1 underwent an unique assembly pathway: while originating from nanospheres similar to previous peptoid tube formation, individual nanotubes were able to entangle and twist to form 3D bundles of nanotubes due to the well-controlled TPE interactions (Figure 3h).
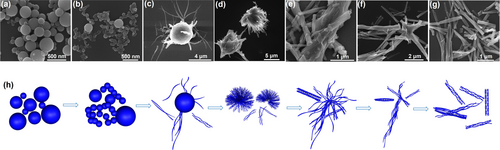
Time-dependent SEM images showing the assembly pathway of PEP-1 nanotube bundles. SEM images of PEP-1 assemblies obtained by dissolved 1 μmol of lyophilized PEP-1 in the mixture of CN3CN and H2O (v/v, 2 : 1) after (a) 5 h, (b) 11 h, (c) 15 h, (d) 20 h, (e) 24 h, (f) 48 h and (g) 72 h, at 4 °C for slow evaporation. (h) Schematic representations show the formation of nanotube bundles.
It is interesting to point out that the assembled nanotube bundles exhibit either left-handed or right-handed helical structures, especially in their initial formation stage (Figure 4a). The assembly of these achiral peptoids into chiral helical bundles of nanotubes is unprecedented, which we believe it is mainly attributed to the interactions among TPE groups. As revealed in the single crystal structure of PEP-10, TPE groups form extensive intermolecular interactions (e.g., π–π stacking) and possess a propeller shape, which can act like gears to interact with each other to engage specific nanotubes. These TPE groups engage in a clockwise or a counterclockwise direction, resulting in the formation of left- or right-handed assemblies, respectively (Figure 4b). Such engagement binds the discrete nanotubes together, generating tube bundles with either left- or right-handed twist (Figure 4c). Using such well-manipulated molecular interactions to build helical hierarchical materials from sequence-defined peptoids provides a new strategy in developing chiral functional materials with controlled chirality and designed properties.
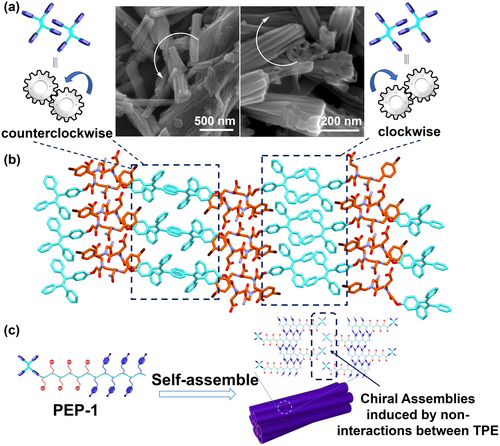
(a) Helical structures of the self-assemblies raised by TPE interactions. (b) Molecular packing of PEP-10 in crystal structure. (c) Cartoon representation of the TPE interactions among peptoid nanotubes to form a right-handed tube bundle structure.
pH-Responsiveness of the Nanotube Bundles Assembled from PEP-1
Benefiting from Nce residues of PEP-1, the pH change can trigger the protonation or the deprotonation of the carboxylic acid group, thereby influencing PEP-1 assembly outcomes and the resulting optical (or fluorescence) properties. Consequently, we investigated the pH-responsiveness of PEP-1 nanotube bundles. For that, PEP-1 nanotube bundles were first dispersed in water with an initial solution pH of 3.75. Both SEM (Figure 5a) and AFM (Figure 5b) images shows PEP-1 assemblies remain the 3D bundle morphology with tightly packed nanotubes. The height of these bundles is around 80 nm (Figure 5b). As solution pH increases, these tube bundles start to swell and become loose. SEM images show some disassociated nanotubes at pH 5.04 and 7.53 (Figure 5c and 5e), while AFM images reveal the height of tube bundles increased to ~120 nm at pH 5.04 and ~300 nm at pH 7.53 (Figure 5d and 5f). Such phenomenon was also observed from the dynamic light scattering (DLS) results which PEP-1 tube bundles showed a size increase upon the increase of solution pH (Figure S16). The increase of the size could attribute to i) the nanotube swelling caused by the charge repulsion of deprotonated Nce groups,6b and ii) the loose packing of nanotubes within bundles. As shown in Figure 5g, SEM image shows that nearly all bundles are untied at pH 10.16. Cracked nanotubes and irregular aggregates are observed at pH 12.98 (Figure 5h), and no tubes are observed at pH 13.72 (Figure 5i). Interestingly, the pH-triggered morphological transformation also changes fluorescence properties of PEP-1 assemblies. As shown in Figure 5j, the fluorescence intensity of PEP-1 assemblies decreased significantly when the solution pH increased from 3.75 to 10.16. It is reasonable because these bundles gradually swell and become loose as the solution pH increases. Consequently, the TPE groups locating on the nanotube surface become loosely packed and exhibit a decrease in fluorescence intensity. Further increase of solution pH leads to the slightly increase of fluorescence intensity (Figure 5j) which could be due to the irregular peptoid aggregation as a results of nanotube collapse.
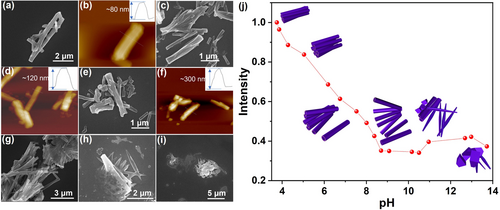
SEM images of PEP-1 nanotube bundles at different solution pH. (a) pH 3.75, (c) pH 5.04, (e) pH 7.53, (g) pH 10.16, (h) pH 12.98, (i) pH 13.72. Ex situ AFM images of PEP-1 nanotube bundles at (b) pH 3.75, (d) pH 5.04, (f) pH 7.53. Insert graph in each AFM image shows the tube bundle height. (j) Fluorescence intensity at 489 nm of the PEP-1 tube bundles at different solution pH: 3.75, 3.86, 4.28, 5.04, 6.18, 6.75, 7.53, 8.01, 8.38, 8.7, 9.08, 10.16, 10.5, 10.96, 12.68, 12.98, and 13.72. The insert cartoons show the morphology of PEP-1 nanotube bundles changes as solution pH increases.
Fluorescence Property of Peptoid Assemblies
By incorporating the TPE fluorophore into assembling peptoids, we have introduced the inherent AIE property of TPE into both peptoids and their assemblies. Consequently, when these peptoid molecules are closely packed, their fluorescence intensity increases, which is confirmed by the increase of fluorescence intensity of PEP-1 or PEP-9 as water fractions increase (Figure S17). Because AIE molecules like TPE are expected to show an enhanced fluorescence intensity when they are closely and orderly packed, thus we hypothesize that the self-assembly of TPE-containing peptoids into crystalline nanomaterials with ordered packing of TPE groups can further increase the fluorescence intensity of these peptoid materials. To test that, we collected the time-dependent fluorescence spectra of peptoid assemblies using PEP-5 and PEP-6. As expected, the fluorescence intensity of peptoid assemblies increased over time (Figure S18a and S18b). The significant fluorescence enhancement can be observed under UV lamp even by naked eyes (Figure S18c), showing the promise of these TPE-containing peptoids in the assembly of highly fluorescent materials.
High-resolution confocal microscopy was employed to image the fluorescent peptoid assemblies. First, the assemblies were diluted in aqueous solution and dropped onto a glass slide. Then the water was removed using filter paper and further dry in the air (see Supporting Information for details). From the high-resolution confocal images, nanotube bundles assembled from PEP-1 and PEP-9 and nanosheet bundles assembled from PEP-3 were clearly observed (Figure 6a–c). All of these assemblies exhibited strong fluorescence, indicating the presence of AIE property. Additionally, liquid cell confocal images of PEP-1 and PEP-9 were obtained to investigate the status of these nanotube bundles in aqueous solution. As shown in Figure 6d and 6e, nanotube bundles assembled from PEP-1 tend to form larger aggregates in solution, while nanotubes assembled from PEP-9 show a more dispersed morphology. The reason could be due to the hydrophobic feature of TPE groups locating on the PEP-1 nanotube surface that promote the aggregation of PEP-1 tube bundles in aqueous solution. In contrast, TPEPy groups on the PEP-9 nanotube surface are positively charged, thus these PEP-9 tube bundles are relatively hydrophilic and can be dispersed in the aqueous solution.
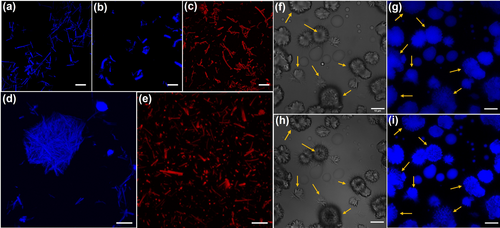
Confocal microscopy images of bundles assembled from (a) PEP-1, (b) PEP-3, (c) PEP-9. (d) PEP-1 nanotube bundles in liquid cell. (e) PEP-9 nanotube bundles in liquid cell. In situ confocal images of PEP-1 assembled in the mixture of CH3CN and H2O (v/v, 2 : 1) in (f,g) position A, 15 h, (h,i) position A, 17.5 h. Scale bar: 10 μm.
Liquid cell confocal imaging was also conducted to directly monitor PEP-1 assembly. Because the most interesting process revealed by the time-dependent SEM images (Figure 3) is the transformation of nanospheres into nanotubes (Figure 3b–3d), we focused our in situ confocal studies on this process. Prior to investigating this transformation process under confocal microscope, PEP-1 solution underwent a 15 h incubation at 4 °C. The transformation process was observed in all three spots (Figure 6f–i and Figure S19). In detail, nanospheres with hairy periphery were observed at 15 h (Figure 6f and 6g), accompanied by some distinctive flower-like assemblies. After incubating for additional 2.5 hours, these nanospheres began dividing into petal-shaped structures, as evidenced in Figure 6h and 6i. Z-stacking images taken from the bottom to the top of these assemblies revealed the formation of pores originating from the inner region of the nanospheres (see Supporting Information for details). This transformation resembles the natural progression of flowers from buds to full bloom. Moreover, structures that had already transformed into “flowers” also exhibited changes: the sharper edges and additional “petals” appeared. Meanwhile, the fluorescence of peptoid assemblies increased significantly over time (Figure S20). This enhancement could be attributed to i) the more ordered packing of peptoid molecules which restricted the movement of the TPE group; ii) the accumulation of fluorescent peptoid assemblies as a result of solvent evaporation. This observed self-assembly process is very interesting and such complex aggregates are rare in pure organic system.1a, 11a, 15 These peptoid assemblies are expected to offer unique opportunities for building functional materials due to the hierarchical multidimensional structures and high crystallinity and tunability.
Artificial Light Harvesting System based on Peptoid Nanotube Bundles
The capability of creating short distance and well-defined orientation of the fluorophore within assembled peptoid nanotubes affords a good platform to construct an artificial light harvesting system through Förster resonance energy transfer (FRET) pathway.4, 8b For this purpose, we selected PEP-1 with TPE and PEP-9 with TPEPy for co-assembly since TPE and TPEPy groups could function as the FRET donor and acceptor, respectively. As shown in Figure 7a, the emission spectrum of PEP-1 solution overlaps well with the absorption spectrum of PEP-9 solution, a prerequisite for constructing FRET systems. The FRET efficiency under different conditions were measured. First, the FRET efficiency was measured in the solution state where the peptoids were amorphous (Figure 7b). Titration of PEP-9 into the solution of PEP-1 resulted in a decrease of PEP-1 fluorescence intensity and a slight increase of PEP-9 fluorescence intensity, indicating a successful energy-transfer between PEP-1 and PEP-9 in solution. Based on the fluorescence titration experiment, the energy-transfer efficiency (ΦET) is calculated to be 70 % when mixing PEP-1 and PEP-9 in solution at a molar ratio of 10 : 1 in the mixture of CH3CN and H2O (v/v, 2 : 8). Then the FRET efficiency of these peptoids with ordered packing was investigated (Figure 7d). Nanotube bundles of PEP-1 and PEP-9 with different molecular ratio were prepared first. From the fluorescence intensity of these co-assemblies, we found that the fluorescent intensity of PEP-1 decreased obviously when the PEP-9 ratio increased. At [PEP-1]/[PEP-9]=10/1, the fluorescence of PEP-1 was barely detectable, and the FRET efficiency was calculated to be 97 %. Through co-assembly of functionalized peptoids, this type of FRET system can be easily fabricated while exhibiting high tunability for selection of various donors and acceptors, as well as an efficient pathway for high energy transfer efficiency. The FRET efficiency observed in this tube bundle system is significantly higher than most of artificial light harvesting systems reported in the aqueous environment,16 and comparable with our recently developed peptoid-based artificial light harvesting systems8b, 17 which we demonstrated that the tubular crystalline structure provides an unique advantage to achieve a high FRET efficiency.17b In contrast, if PEP-1 and PEP-9 were assembled individually to form nanotube bundles and then mixed together, this resulting FRET system showed a low efficiency of 60 % at [PEP-1]/[PEP-9]=10/1 (Figure 7e). The reason could be that the fluorophores (i.e., TPE, TPEPy) in the assembled tube bundles were densely packed, leading to the limited contact between TPE and TPEPy. This phenomenon also suggested the high stability of assembled crystalline tube bundles. The fluorescence of these tube bundles co-assembled from PEP-1 and PEP-9 was further investigated using confocal microscopy. With the increase of PEP-9 ratio, the fluorescence of the co-assemblies at channel ranging from 415 to 501 nm (channel 1) gradually quenched, while the fluorescence ranging from 551 to 705 nm (channel 2) lighted up (Figure S21). If the assembled PEP-1 and PEP-9 were mixed together, both fluorescence at channel 1 and channel 2 can be detected (Figure S21). Lambda scan images of different co-assemblies showing the fluorescence intensity in different wavelength were obtained (Figure S22–S28). As a result, the fluorescence of the co-assemblies red-shifted from 439–595 nm to 537–673 nm with the increasing of PEP-9 ratio (Figure S29). All these results confirmed that the assembly of TPE- and TPEPy-containing peptoids into crystalline tube bundles is a successful strategy to develop a highly efficient light harvesting system in the aqueous environment.
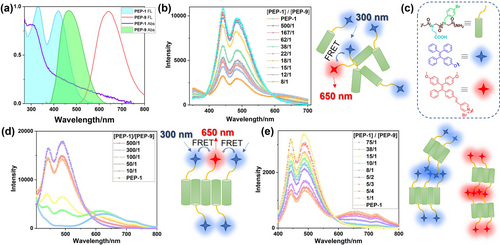
(a) Normalized emission and absorption spectra of PEP-1 and PEP-9 solutions. (b) Fluorescence spectra of PEP-1 in the mixture of CH3CN and H2O (v/v, 2 : 8) with different ratios of PEP-9, and the illustration of the FRET process in solution. λex=300 nm, [PEP-1]=1.2×10−4 M. (c) Chemical structures and schematic representations of the building blocks. (d) Fluorescence spectra of the co-assembled PEP-1 and PEP-9 at different ratios, and the illustration of the FRET process in the co-assemblies. λex=300 nm, [PEP-1]=6×10−5 M. (e) Fluorescence spectra of the assembled PEP-1 with different ratios of assembled PEP-9, and the illustration of the FRET process in the mixture of individual assemblies. λex=300 nm, [PEP-1]=2×10−5 M.
Conclusion
In conclusion, by manipulating the molecular interactions among functional peptoids, we developed a variety of peptoid-based crystalline nanomaterials with multidimensional structures, including 3D nanotube bundles. We showed that the choice of peptoid sequences plays a crucial role in dictating molecular interactions and the assembly outcomes to generate various nanostructures. By obtaining the crystal structure of peptoids and investigating the molecular interactions among specific functional groups and side chains, we gained molecular level understanding of the peptoid assembly mechanisms and the formation pathways of peptoid nanotube bundles. We found that the molecular interactions among TPE aromatic groups are important for controlling the formation of 3D twisted bundles of peptoid nanotubes or nanosheets, while interactions among the non-polar domains of assembling peptoids determine the formation of either nanotubes or nanosheets. Through pH control and variation of side chains in the assembling peptoids, we further demonstrated the pH-triggered responsiveness of these nanotube bundles and achieved the tunable fluorescent properties. Furthermore, we showcased the creation of a highly efficient artificial light harvesting system in the aqueous environment using these peptoid nanotube bundles. Because peptoids are biocompatible and high tunable, we expect this FRET system exhibits great potential for a range of biomedical applications, including cell imaging and photodynamic therapy. Overall, our work introduces a novel strategy of using well-controlled molecular interactions to construct functional materials from sequence-defined synthetic polymers. It also provides molecular level insights into the intricate relationship between the molecular structures of synthetic polymers and the resulting macroscale assemblies.
Acknowledgments
This work was mainly supported by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences (BES) as part of the Energy Frontier Research Centers program: CSSAS—The Center for the Science of Synthesis Across Scales—under Award Number DE-SC0019288 [FWP 72448 at Pacific Northwest National Laboratory (PNNL)]. The synthesis of peptoid nanosheets was supported by DOE-BES biomolecular materials program under an award FWP 65357 at PNNL. The synthesis and assembly of PEP-al, DLS and CD studies were supported by DOE-BES under an award FWP 80124. XRD work was conducted at the Advanced Light Source (ALS) of Lawrence Berkeley National Laboratory, which was supported by the Office of Science (No. DE-AC02-05CH11231). PNNL is multi-program national laboratory operated for DOE by Battelle under Contracts No. DE-AC05-76RL01830.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




