Total Synthesis of Lobatamides A and C
Abstract
The total synthesis of lobatamides A (1 a) and C (1 c) via a common bislactone intermediate is reported. The allylic aryl moiety including a trisubstituted Z-olefin was constructed by hydroboration of a 1,1-disubstituted allene and subsequent Migita-Kosugi-Stille coupling. Although the seco acid proved to be highly unstable even in the presence of weak bases, Zhao macrolactonization under acidic conditions via the α-acyloxyenamide successfully provided the common bislactone intermediate. Hydrozirconation-iodination of the terminal alkyne and subsequent copper-mediated coupling with primary amides proceeded successfully in the presence of the sensitive bislactone framework. The developed synthetic route enables the late-stage installation of enamide side chains, which are crucial structures for V-ATPase inhibition.
Salicylate enamide natural products1 such as lobatamide A (1 a),2a, 2c salicylihalamide A (2)3 and apicularen A (3)4 share a unique structure comprised of a central macrobenzolactone and an enamide side chain (Figure 1). This class of natural products is known to inhibit mammalian vacuolar-type proton ATPase (V-ATPase). V-ATPase is an ATP-driven proton pump, play a crucial role in intracellular pH regulation, and has been studied as a promising drug target for cancer therapy.5 Salicylate enamides have been identified as promising lead compounds for antitumor agents because proton-extruding V-ATPase is expressed on the plasma membranes of human tumor cells.5a, 5b Among these salicylate enamides, lobatamides2 are recognized as the most structurally complex natural products known (Figure 1A). The Boyd group isolated lobatamides A (1 a) and B (1 b) from a southwestern Pacific tunicate Aplidium lobatam.2a Soon after, they reported isolation of a series of lobatamides including lobatamide C (1 c).2a, 2c Interestingly, the Suzumura group independently reported the isolation of YM-75518A-D (1 a–d) from the fermentation broth of Pseudomonas sp. Q38009 (Lobatamides A-C were identical to YM-75518A-C, respectively).2b, 2d The Boyd group reported that the lobatamides showed antiproliferative effects against 60 human cancer cell lines tested in the National Cancer Institute (NCI) with a mean panel GI50 value of approximately 1.6 nM.2c They found that their antiproliferative effects were caused by the inhibition of mammalian V-ATPases.6 In 2002, the Porco group reported the first total synthesis of lobatamide C (1 c) by copper-mediated enamidation, and determined its relative and absolute configurations.7 They also prepared simplified analogues and performed V-ATPase inhibition studies, showing the important role of the enamide side chain.7b, 8 Their total synthesis of lobatamide C (1 c) has been recognized as a milestone of studies related to salicylate enamide natural products.
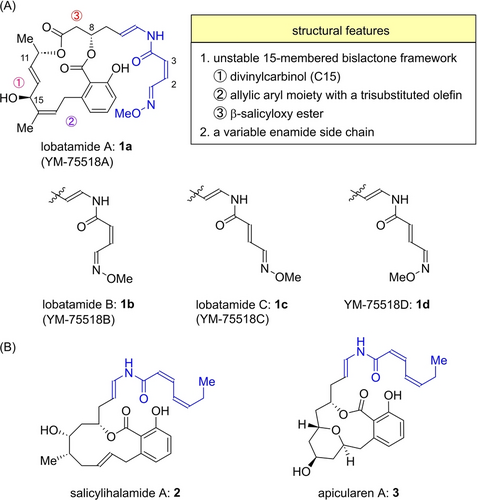
Structures of lobatamides.
One of the remaining challenges in the synthesis of lobatamides is the development of a universal route to give quick access to a variety of lobatamides, which possess the common bislactone framework and a variable enamide side chain (Scheme 1). Considering the biological importance of the enamide side chains, we envisioned the late-stage diversification of enamide side chains from common bislactone intermediate 9. For a convergent synthesis, common intermediate 9 was divided into three fragments including divinylcarbinol 4, aryl stannane 6, and carboxylic acid 8. Divinylcarbinol 4 would be synthesized by stereoselective allenylation of an α,β-unsaturated aldehyde. Previously, we developed a stereodivergent approach to give access to allylic aryl structures containing a trisubstituted olefin.9l Thus, the method including Z-selective hydroboration of the 1,1-disubstituted allene 49 and subsequent Migita-Kosugi-Stille coupling with aryl stannane 610 would form the crucial allylic aryl framework. Intermolecular esterification with carboxylic acid 8, followed by macrolactonization, would provide common bislactone intermediate 9. Considering that lobatamides possess enamide side chains with various types of stereochemistries, it is reasonable to employ the late-stage enamidation from common bislactone 9 and primary amide 10. The main synthetic issue in the total synthesis of lobatamides is the instability of these natural products due to several sensitive functional groups under either acidic or basic conditions.2c, 7 The divinyl carbinol at the C15 carbon center could be easily liberated as a leaving group by the resonance effect. The allylic aryl moiety might undergo isomerization to the conjugated styrene-type structure. The β-salicyloxy ester, which is not found in other salicylate enamides, is especially troublesome due to facile β-elimination.7 Therefore, the late-stage installation of the enamide side chains into the sensitive common bislactone is highly challenging. In this communication, we report the total synthesis of lobatamides A (1 a) and C (1 c) by the late-stage installation of enamide side chains through hydrozirconation/iodination of an alkyne, and subsequent copper-mediated coupling with primary amides.
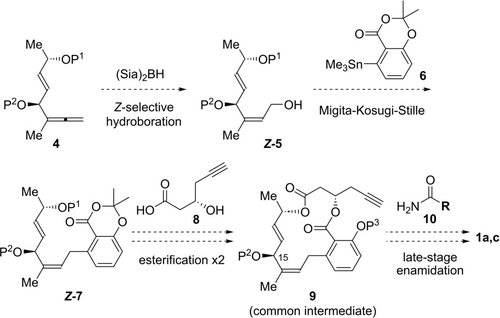
Synthetic plan toward the total synthesis of lobatamides. Sia=1,2-dimethylpropyl.
Our total synthesis commenced with diastereoselective allenylation of α,β-unsaturated aldehyde 12, which was prepared from lactate ester 11 in a known three-step sequence11 (Scheme 2). Although the diastereselective allenylation of 12 proved to be not trivial, Chen's procedure using the propargylic boron ester in the presence of a chiral phosphoric acid12 provided divinylcarbinol 13 in 92 % yield as a single diastereomer. The resulting secondary alcohol was protected as a TIPS ether to give 1,1-disubstituted allene 4, which was used for the synthesis of allylic aryl moiety Z-7. Thus, hydroboration of 1,1-disubstituted allene 49k, 9l with (Sia)2BH and oxidative work-up provided allylic alcohol Z-5 in 85 % yield with almost complete Z-selectivity. Mechanistically, (Sia)2BH approached allene 4 from the sterically less hindered methyl side (Scheme 3). The resulting oxidative work-up of Z-allylic borane Z-15 resulted in the formation of Z-5. The key to success was utilization of sterically large (Sia)2BH, which prevented 1,3-allylic rearrangements via 1613 to give thermodynamically favorable E-5. In fact, when using sterically less hindered 9-BBN, E-5 was obtained in 43 % yield (Z : E=1 : 13). We also expected that the large TIPS group on the secondary alcohol of 4 would distinguish it sterically from the methyl group, in addition to its robustness as a protecting group. Subsequent Migita-Kosugi-Stille coupling with aryl stannane 614 was not trivial due to the facile isomerization of the Z-isomer to the E-isomer via the π-allylic Pd species (Scheme 2).15 For example, coupling conditions using the allylic methyl carbamate with aryl stannane 6 in the presence of Pd2(dba)3⋅CHCl3 and LiCl in DMF at 80 °C9l caused significant isomerization, giving E-7 as the major diastereomer (37 %, Z : E=1 : 6.9). After extensive investigation (see the Supporting Information), we found appropriate conditions to suppress this isomerization. First, allylic alcohol Z-5 was converted to allylic chloride 14 with p-TsCl and DMAP. The resulting 14 was subjected to the coupling reaction with 6 in the presence of 10 mol % Pd(PPh3)4, CuTC and NaI in NMP, giving Z-7 in 80 % yield (Z : E=11 : 1).15b
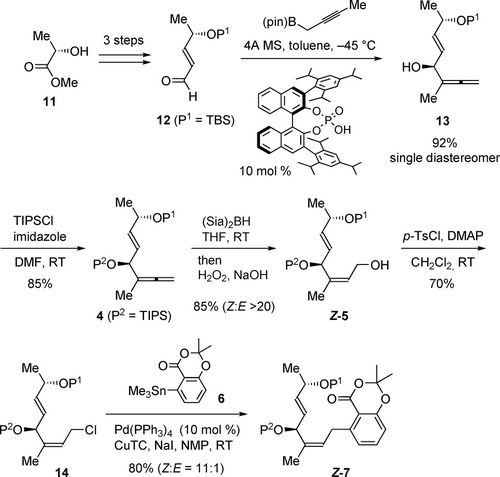
Synthesis of the allylic aryl moiety. DMAP=N,N-4-dimethylaminopyridine, MS=molecular sieves, NMP=N-methyl-2-pyrrolidinone, pin=pinacol, TBS=tert-butyldimethylsilyl, TC=2-thiophenecarboxylate, TIPS=triisopropylsilyl, Ts=toluenesulfonyl.
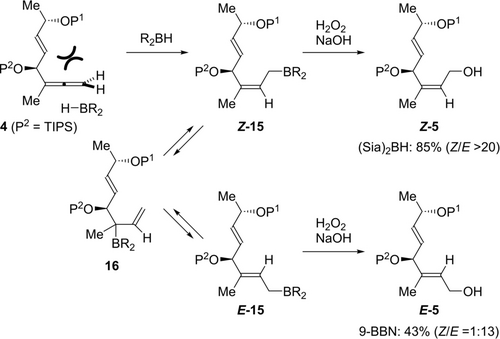
Stereodivergent hydroboration of 1,1-disubstituted allene 4.
Having the stereoselective synthesis of the allylic aryl moiety established, we turned to the assembly of the bislactone skeleton (Scheme 4). After hydrolysis of 7 with TMSOK, intermolecular esterification of the resulting salicylic acid 17 was achieved by modified Porco's conditions.7 First, salicylic acid 17 was converted to cyanomethyl ester 18 using ClCH2CN and Et3N at reflux. Transesterification of 18 with β-hydroxy acid 816 proceeded in the presence of NaOAc and 4A MS at 60 °C to give β-salicyloxy acid 19. Selective cleavage of the TBS group in 19 with HF⋅pyridine provided seco acid 20, which was subjected to a variety of macrolactonization conditions. However, promising methods such as Yamaguchi conditions (2,4,6-trichlorobenzoylchloride)17 and Shiina conditions (MNBA)18 did not produce bislactone 22 due to the instability of the β-salicyloxy acid. Indeed, exposure of seco acid 20 to weak bases such as Et3N and iPrNEt2, which are widely used in various macrolactonizations, resulted in decomposition. Finally, we found that acid-catalyzed macrolactonization developed by Zhao19 solved this instability issue. First, seco acid 20 was converted to α-acyloxyenamide 21 with N-methyl ynetoluenesulfonamide (71 % for 2 steps). Originally, Zhao reported that the best acid in the cyclization was p-TsOH, but significant decomposition was observed in our case. However, addition of 45 mol % of CSA to 21 successfully promoted the macrolactonization, giving bislactone 22 in 33 % yield. It is noteworthy that bislactone 22 was found to be gradually decomposed in concentrated form, thus it was immediately used for the subsequent TES-protection of the remaining phenol to give relatively stable common intermediate 9.
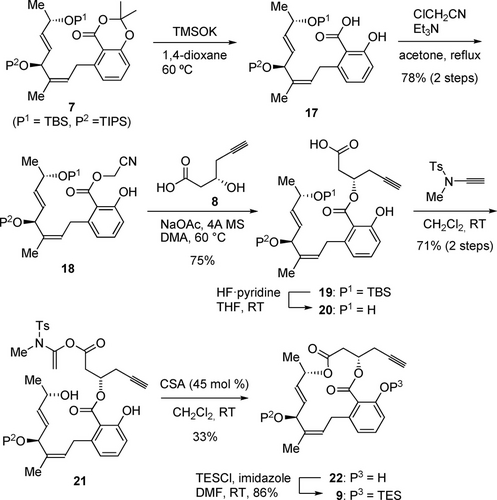
Synthesis of the common bislactone intermediate. CSA=10-camphorsulfonic acid, DMA=N,N-dimethylacetamide, DMF=N,N-dimethylformamide, TES=triethylsilyl, TMS=trimethylsilyl.
With common intermediate 9 in hand, the stage was set for the crucial late-stage installation of the enamide side chains,20 which required reaction conditions that did not affect the sensitive β-salicyloxy ester and generated enamide groups (Scheme 5). After extensive studies including electrophilic enamidations,21, 22 hydrozirconation of the terminal alkyne23 followed by iodination was found to give E-iodoolefin 23 in 87 % yield. When applying modified Buchwald conditions,24, 25 copper-mediated coupling of 23 with primary amide (E,E)-107 in the presence of diamine ligand 24 and K3PO4 successfully installed the enamide side chain without scrambling the stereochemistry, giving enamide (E,E)-25 in 33 % yield. The two silyl groups in (E,E)-25 were carefully cleaved with HF⋅pyridine at 60 °C. Finally, purification by reverse phase HPLC afforded the pure sample of lobatamide C (1 c) in 50 % yield.26 The developed sequence proved to be reliable, and could be applied to the synthesis of lobatamide A (1 a). The copper-mediated coupling reaction of E-iodoolefin 23 with primary amide (Z,E)-107 gave enamide (Z,E)-25 in 46 % yield without causing isomerization of the Z-olefin. Careful deprotection with HF⋅pyridine and subsequent purification by reverse phase HPLC provided lobatamide A (1 a) in 23 % yield.26 Thus, we accomplished the first total synthesis of lobatamide A (1 a). The stereochemical assignment of lobatamide A (1 a) was confirmed by our total synthesis as identical to lobatamide C (1 c) except for the C2−C3 Z-olefin. While the Boyd group reported the NMR spectra of lobatamide A (1 a) in MeOH-d4, the Suzumura group documented the NMR spectra of 1 a in DMSO-d6. Thus, our synthesis also confirmed that their isolated samples of lobatamide A (1 a) were actually identical.
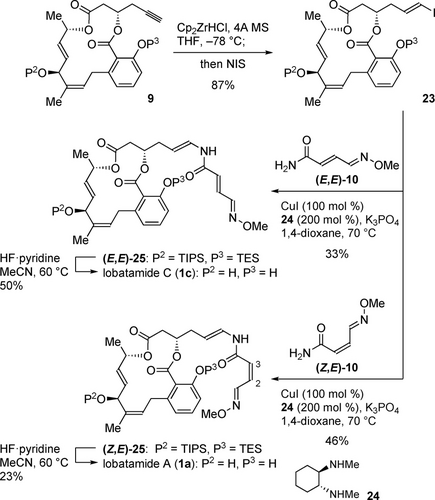
Total synthesis of lobatamides A and C. Cp=cyclopentadienyl, NIS=N-iodosuccinimide.
In conclusion, we developed a unified route toward the total synthesis of lobatamides A (1 a) and C (1 c). Construction of the allylic aryl moiety contained a number of intriguing steps, such as stereoselective hydroboration of a 1,1-disubstituted olefin and palladium-catalyzed coupling to suppress the E/Z-isomerization of the trisubstituted olefin. The keys to success in the total synthesis were the judicious choice of reaction conditions without affecting sensitive functional groups, as seen in the Zhao macrolactonization to give the common bislactone intermediate, and the late-stage installation of an enamide side chain. Our synthesis will enable the diversification of the enamide side chain, which is known to play an important role in the biological activity of these natural products. Structural activity relationship studies involving V-ATPase inhibition for investigating a new class of antitumor agents is ongoing.
Acknowledgments
This research was supported by JST SPRING (JPMJSP2123) for S.Y. and S.B. We thank Prof. Makoto Inai and Prof. Fumihiko Yoshimura (the former Kan group, University of Shizuoka) for the critical advice for the copper-mediated coupling reaction in the total synthesis. We are grateful to Prof. Suenaga and Dr. Kurisawa for useful advice on purification of lobatamides. We thank Prof. Ryuichi Sawa, Prof. Masayuki Igarashi, and Yumiko Kubota (Institute of Microbial Chemistry) for the NMR measurement of 1 a.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




