Spontaneous Oxidation in Aqueous Microdroplets: Water Radical Cation as Primary Oxidizing Agent
Abstract
Exploration of the unique chemical properties of interfaces can unlock new understanding. A striking example is the finding of accelerated reactions, particularly spontaneous oxidation reactions, that occur without assistance of catalysts or external oxidants at the air interface of both aqueous and organic solutions (provided they contain some water). This finding opened a new area of interfacial chemistry but also caused heated debate regarding the primary chemical species responsible for the observed oxidation. An overview of the literature covering oxidation in microdroplets with air interfaces is provided, together with a critical examination of previous findings and hypotheses. The water radical cation/radical anion pair, formed spontaneously and responsible for the electric field at or near the droplet/air interface, is suggested to constitute the primary redox species. Mechanisms of accelerated microdroplet reactions are critically discussed and it is shown that hydroxyl radical/hydrogen peroxide formation in microdroplets does not require that these species be the primary oxidant. Instead, we suggest that hydroxyl radical and hydrogen peroxide are the products of water radical cation decay in water. The importance of microdroplet chemistry in the prebiotic environment is sketched briefly and the role of partial solvation in reaction acceleration is noted.
1 Introduction
Recent investigations into chemical reactions in microdroplets offer promise of changing this situation dramatically.3-8 The key feature of microdroplet chemistry is acceleration of reaction by an impressive 2 to 6 orders of magnitude. This remarkable phenomenon is associated with three critical characteristics of the gas/solution interface: (i) reduced solvation at the interface, which increases the frequency of bimolecular collisions between reactants,9 (ii) the existence of a high electric field (∼109 V/m) at the interface,10, 11 presumably leading to improved molecular orientation and increased successful collision rates,12 and (iii) the associated presence of highly reactive redox species, such as water radical cation. The significance of gas/solution interfacial droplet chemistry is becoming increasingly apparent with numerous examples of oxidation in particular.8, 13-16 This discussion argues that this chemistry finds its origin in the water radical cation which is the primary reactant (together with the corresponding anion) at aqueous and mixed aqueous organic interfaces.
This minireview offers a summary of the chemistry of water radical cations across diverse environments, spanning the gas phase, solution, and the microdroplet gas/solution interface. It critically examines the distinguishing attributes of the microdroplet interface, elucidating the connection between its unique features and oxidation reactions at this interface. Furthermore, it presents a fundamental mechanism for spontaneous oxidation in microdroplets, proposing a central role for water radical cation/anion pairs as the primary chemical source of redox properties. This general mechanism reconciles different interpretations of experimental results, holding promise for resolving the ongoing debate surrounding the nature of the reactive oxidative species in microdroplet chemistry.
2 Water Radical Cations in the Gas Phase and in Solution
2.1 Generation and Observation of Water Radical Cations
Gas-phase monomeric water radical cation, H2O+⋅ is generated readily by direct ionization of water vapor. This was done in vacuum in 1923, when Smyth observed an unexpected ion in a mass spectrum at m/z 18 and attributed it to H2O+⋅, resulting from electron impact on water vapor.17 Subsequent research using mass spectrometry has characterized H2O+⋅ in vacuum including its ionization energy and ion/molecule reactivity.18-23 However, spectroscopic evidence for this species remained scarce until 1973 when Heiber et al. reported both absorption and emission features in the range 350–680 nm.24
Studies of water radical cations under ambient conditions are more relevant to microdroplet reactivity. Recently, Chen et al. developed a corona discharge-based device for the generation of water radical cations in air in quantity (Figure 1a).25 By introducing a moist inert gas into the discharge volume, direct ionization of water was achieved in air and this allowed detection of water cations by mass spectrometry. Intact H2O+⋅ ions were accompanied by a dominant mono-solvated ion, H2O+⋅(H2O) and by higher solvated forms (Figure 1b). The relatively high abundance of the water dimer radical cation hints at its exceptional stability compared to the polysolvated species. Various computational studies have proposed two contributing structures for the charged water dimer.26-28 One isomer exhibits characteristics of hydrogen O−H bonding (H3O+…⋅OH structure A in Figure 1c). The other isomer, H2O+⋅…OH2, contains a one-electron O−O bond (structure B in Figure 1c). Computations indicate that structure A is more stable by 8.8 kcal/mol vs. isomer B. Moreover, the calculation highlights a 15.1 kcal/mol energy barrier to their interconversion (Figure 1c).26 While experimental evidence is limited and somewhat indirect; the existence of both structures, A25, 27, 28 and B25, 29, 30 has been reported, consistent with their related but distinctive reactivities.25 Further, the hemi-bonded isomer was verified as the intermediate complex in the proton transfer between H2O+⋅ and H2O.28 Notably, recent research further validated the interconversion using a linear ion trap mass spectrometer.31

Water radical cation formed under ambient conditions. (a) Generation of water radical cation by corona discharge. (b) Mass spectrum of generated water ions in the positive mode. Inset: High resolution mass spectrum of (H2O)2+⋅. Figure 1a and 1b are reproduced with permission.25 Copyright 2021 Chinese Chemical Society. (c) Structures of water dimer radical cations and their interconversion. Reproduced with permission.26 Copyright 2009 American Chemical Society.
One noteworthy property of (H2O)2+⋅ is its lifetime, which is intimately linked to the chemical environment. For instance, (H2O)2+⋅ can persist in an ion trap for at least 200 ms, and the monomeric form exhibits an even longer lifetime, lasting for at least 800 ms.31 This differs notably from the lifetime of water radical cation in aqueous solution (less than a few hundred femtoseconds).28, 32, 33 Meanwhile, in solution, the solvation behavior of water radical cation differs between monomeric and dimeric forms. Hence, a thorough exploration of water radical cation's chemical reactivity in different scenarios is indispensable for understanding its role as an oxidant and for harnessing the potential of this highly reactive species.
2.2 Chemical Reactions of Water Radical Cations
Ion-molecule reactions between water radical cation and polyatomic molecules (M) were first investigated in vacuum.18, 19 Three types of reactions are involved (Figure 2, top panel), namely (I) electron transfer (also called charge transfer) from the neutral molecule to H2O+⋅ to form a molecular ion (M+⋅) and seen for NO, O2 and CH2O, (II) proton transfer from H2O+⋅ to the neutral, generating the protonated molecule ([M+H]+) and hydroxyl radical as products, as seen for neutral water itself, and (III) hydrogen atom transfer from the neutral to H2O+⋅, e.g. CH4, to give H3O+⋅. It is notable that the three reaction channels are not mutually exclusive, for example, the reaction between H2O+⋅ and H2S generates H2S+, H3S+ and HS as products.19
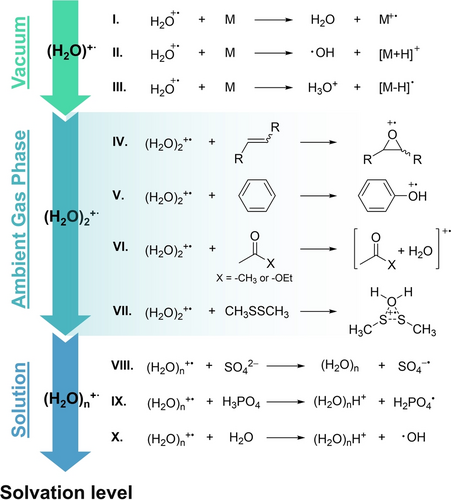
Summary of reactions involving water radical cations in vacuum, ambient gas phase (wet air), and in solution. As the solvation level of the environment increases so does the number of water molecules associated with the water radical cation, while the reaction scope becomes more limited.
Under humid ambient conditions, the water dimer radical cation is the dominant species (Figure 1b). The types of reaction of (H2O)2+⋅ are similar to those of the monomer in that electron transfer and proton transfer occur.34 Most notably, its chemistry revolves around the direct oxidation of unsaturated chemical bonds (Figure 2, middle panel), including alkene,35 acetone,36 ester,25 and benzene,25, 37 as confirmed by exact mass measurements35, 37 and 18O-labeled experiments.25, 35-37 It is worth noting that the true structure of the intermediate (M+H2O)+⋅ in the oxidation of carbonyl compounds remains undetermined, although a reasonable case is made for a geminal diol radical cation. In the case of benzene, oxygen insertion occurs to give phenol as oxidation product, with an proposed intermediate, (C6H6…H2O+⋅).25, 37 The reaction of disulfides with (H2O)2+⋅ also results in the formation of (M+H2O)+⋅, presumably adopting a triangular conformation, as illustrated in Figure 2, reaction VII.25 The gas-phase chemistry of water radical cation has been thoroughly reviewed.38 An unresolved question regarding the interconversion between the proton-bonded dimer (A) and the hemi-bonded dimer (B), concerns which of these structures accounts for the observed oxidation. Notably, benzene oxidation is attributed to structure A, while disulfide oxidation is attributed to structure B.25 It is notable, too, that water radical cations at various solvation levels, such as monomer, trimer or those with a higher water content as depicted in Figure 2b, may also be involved in the oxidation process. This is particularly relevant considering that the dimer is not isolated in the experimental environment.
The solution-phase chemistry of the water radical cation in solution is illustrated in Figure 2, bottom panel. Although the generation of water radical cations is considered as the initial step in the radiolysis of water,39 direct observation of the oxidation in aqueous solution presents a substantial challenge due to the ultrafast decay of water radical cations upon interaction with the solvent, water (Figure 2, reaction X).28, 32, 33 The lifetime of the monosolvated water radical cation is not known, although it is expected to be considerably longer. Hence, water-deficient environments proximate to the water radical cation are pivotal in decreasing undesired reactions. This expectation is consistent with the observation that the oxidation of H2SO4 and H3PO4 by water radical cations, generated through water radiolysis, is only seen in concentrated solutions (Figure 2, reactions VIII and IX),40, 41 where the water radical cation primarily encounters the reactants (H2SO4 and H3PO4) rather than water itself. Encounters with water result in the generation of ⋅OH, a milder oxidant incapable of oxidizing either of these acids. The slow proton transfer between the water radical cation and these strong acids further facilitates oxidation processes by the water radical cation.
3 Water Radical Cations in Microdroplets
3.1 Unique Interfacial Properties of Microdroplets
Water radical cation is considered the strongest oxidant in aqueous solutions, with an estimated standard redox potential (H2O+⋅/H2O) exceeding 3 V.33, 42 However, its practical application is hampered by two key limitations: (i) the high energy required for its generation and (ii) the rapid reaction of the water radical cation in the presence of proton acceptors, like water itself. A recent perspective by Ben-Amotz has shed a light on this dilemma, proposing that the charged water pair (H2O+…H2O−) exists fleetingly in bulk water at a concentration of 3 M.2 The pair is generated through initial electron delocalization along the hydrogen bond between two adjacent water molecules (which gives the high concentration but a short lifetime), followed by the electron transfer event. A calculation suggests that this process can be significantly enhanced at the interface where an asymmetric hydrogen bond breaks, facilitating charge delocalization.43 Small aqueous microdroplets, with their relatively large interfacial areas, are therefore ideal for fostering the generation of water radical cations. Furthermore, microdroplet surfaces have unique and interconnected features including (i) strong electric field, (ii) highly acidic environment, (iii) partial solvation of reagents, (iv) low water content, and (v) high local concentration of redox species (Figure 3), which support the generation of water radical cations and promote the desired oxidation reactions, while simultaneously reducing the undesired decay of these reactive species.
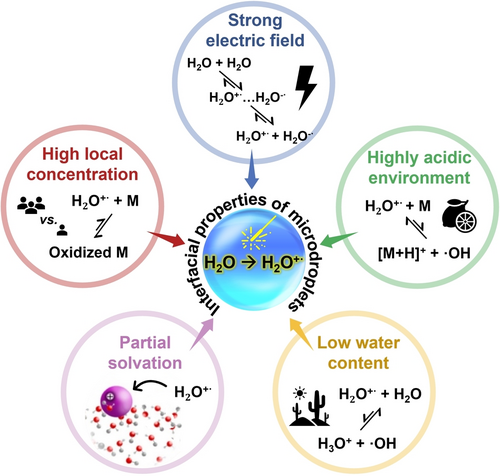
Five interfacial properties of microdroplets that control spontaneous oxidation driven by water radical cation.
Microdroplets can function as electrochemical cells, owing to the presence of strong electric fields at their surfaces. Electric field formation relies on (i) alignment of water molecules, with dangling O−H bonds oriented perpendicular to the surface,10 and/or (ii) electron transfer leading to a water radical pair. An overall net charge may occur and it may be the result of (i) ionic gradients, where ions such as bicarbonate accumulate near the surface, resulting in the buildup of an electrical double layer,44 or (ii) it may be produced during electrospray45, 46 or sonic spray microdroplet generation.47 Spectroscopic techniques have been employed to quantify the electric field strength, and they reveal a remarkably high field of approximately 109 V/m at aqueous microdroplet surfaces (although the interface is water-oil).11 It seems that the strong electric field can facilitate the generation and persistence of water radical cations through the separation of charged water pair (H2O+…H2O−) induced by the electric field. It is notable that direct ionization of water can also form the water radical cation. Note too, that this latter process requires far less energy than direct ionization of isolated water, which has the relatively high ionization energy of 12.6 eV.48
Meanwhile, consideration of this equation prompts a novel inquiry: Could the water radical cation serve as a potential chemical source for the lower pH at or near the interface? Although the answer is still under investigation, we believe eq. 3 is the key to connecting Brönsted's equation with Ben-Amotz's equation (eq. 2).
The rapid decay of water radical cation (eq. 3) raises a new question: how can the water radical cation survive in aqueous droplets? The answer is simple: surfaces of charged aqueous microdroplets are relatively dry. The local environment is rich in charged species (e.g. proton, water radical cation, hydronium ion) but is relatively water-poor. This situation offers an intriguing solution to a longstanding enigma in the field of the origin of life—the water paradox.51 Specifically, it addresses the question of how primordial biomolecule precursors, such as amino acids, can undergo dehydration to give rise to biopolymers like peptides.52 The “dry” surface conditions also play a crucial role in preserving water radical cations at the surface, as they experience reduced interactions with water, thereby diminishing their loss by hydration reactions that ultimately lead to hydronium ions and hydroxyl radicals as well as further secondary products like hydrogen peroxide.
The qualitative concept that the surfaces of aqueous microdroplets are relatively dry is closely linked to another concept, namely the partial solvation hypothesis, which is now discussed. The overwhelming importance of solvation in reaction kinetics is well-known from the epochal work of Brown, Brauman and others.53, 54 It is generally understood to account for the orders-of-magnitude difference in reaction rates between the gas phase and the bulk solution.55 This fact is particularly significant in explaining the difference in bulk solution and microdroplet reaction rates. Partial solvation of reactants at the droplet interface explains why reaction rates exhibit a remarkable acceleration, reaching values of 104 to 106.4, 9 Much like the molecular mechanism for surface tension,56 the active species located at the surface experience limited interactions with a reduced number of solvent molecules. This phenomenon leads to the incomplete envelopment of reactants by their solvation shells, thereby altering the reaction energy profile and producing a reaction rate intermediate between those in the gas phase and bulk solution. This general mechanism is believed to accelerate oxidation by water radical cation as well.
By analogy to the oxidation of H2SO4 and H3PO4 in solution, a high concentration of reactants proves to be essential for their oxidation by water radical cation. In microdroplet synthesis, solvent evaporation naturally boosts reactant concentrations,57 while the accumulation of surface-active compounds further contributes to increased surface concentrations.58, 59 These dual mechanisms function in tandem to ensure that surface-generated water radical cations promptly engage with substrate M in its oxidation.
3.2 Spontaneous oxidation in microdroplets
Similar to ambient gas phase chemistry, reactions between the water radical cation and substrate (M) in microdroplets begin with direct addition (Figure 2, reactions IV to VII). When ions of organic compounds are generated via electrospray (with accompanying microdroplet formation), the protonated form of the neutral molecule (M+H+) typically predominates. Nevertheless, when the compound contains a heteroatom double bond, conditions favoring the dominance of the (M+18) ion become apparent, with two possibilities: either the simple ammonium adduct (M+NH4+) or water radical cation adduct (M+H2O+⋅). In fact, in an extensive study involving approximately 21,000 multifunctional compounds, a notable 18 % of all compounds exhibited abundant (M+18) ions, with this proportion increasing to 65 % within the subgroup featuring X=Y bonded compounds.60 This preference agrees with the observed trend in the gas phase (Figure 2, middle panel), supporting the existence, or at least, partial existence, of (M+H2O+⋅) in those cases, although further experiments to discriminate between the two possible species are required. What is particularly interesting is the possible close connection of the (M+H2O+⋅) ion to the spontaneous redox reactions. The ion H4O2+⋅ has been successfully detected in spray-based microdroplets using mass spectrometry.14, 60, 61 Although the direct evidence to show (M+H2O+⋅) conversion to the final oxidation products is missing, the addition of water dimer radical cation was shown to improve the oxidation conversion (see section 3.3).60, 62
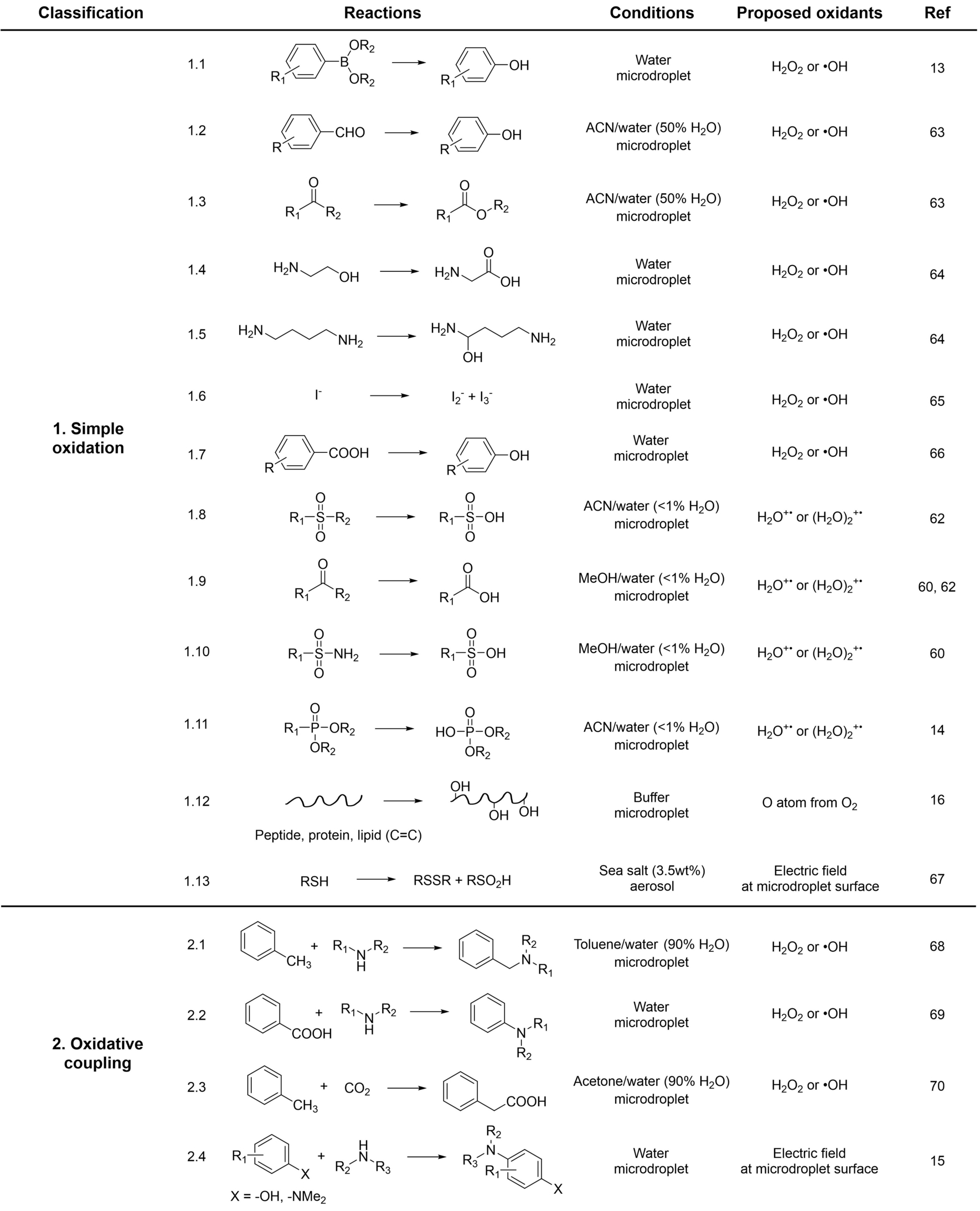
The true identity of the oxidizing agent is a widely-discussed yet controversial subject in microdroplet chemistry. Table 1 summarizes the classification, reaction conditions, and oxidants suggested in various studies. Generally, two types of oxidation have been observed in microdroplets: (i) simple oxidation of substrates, where a single reactant is oxidized by an oxidant to yield the final product,13, 14, 16, 60, 62-67 and (ii) oxidative coupling reactions, in which one reactant is oxidized to generate a radical species (as intermediate) that subsequently couples with another reactant to produce a coupling product.15, 68-70 As shown in table 1, spontaneous oxidation encompasses a wide array of compounds, from organic compounds (most cases), through inorganic compounds (reaction 1.6) to large biomolecules (reactions 1.12 and 1.13).
It is notable that spontaneous reduction processes have also been observed in microdroplets,71-75 encompassing small molecule reduction and the formation of metal nanoparticles. In some instances, these reduction occur concurrently with oxidation,14, 76 indicating the coexistence of both oxidants and reductants. Much like the ongoing debate surrounding oxidation, the identity of reductants and their sources remain under investigation. The hydroxide at the droplet surface might be an electron donor, while the water radical anion (viz. the hydrated electron) is possibly a more plausible reducing agent. Although this is consistent with the importance of the redox pair discussed above (eq. 2), reduction is not a focus of this review.
3.3 Primary Oxidants in Microdroplet Reactions
An intriguing finding shown in Table 1 is the diversity of suggested oxidants, including (i) hydrogen peroxide and/or hydroxyl radical, often hypothesized to arise from the oxidation of hydroxide at the microdroplet‘s interfacial electric field, (ii) water radical cation (in a monomeric or dimer form), believed to originate from water radical pairs separated by the electric field, (iii) oxygen atoms, derived from molecular oxygen in the surrounding air due to the electric field, and (iv) direct electron transfer by the electric field itself. There is a broad consensus that the interfacial electric field serves as the primary source of oxidative activity. However, discriminating between direct electron detachment by the electric field from indirect oxidation via chemical oxidants generated within (or responsible for) the field remains a significant challenge. Even the investigators who suggest that reactions 1.13 and 2.4 are directly electric field driven, as supported by evidence of the inability of hydrogen peroxide to oxidize the substrates and the ability of electric field to direct oxidize the substrates (as estimated based on oxidation potentials), do not conclusively eliminate alternative chemical oxidants. Another interesting finding emerges from comparison of reactions 1.3 and 1.9, where both reactants possess with the same functional group but yield different products—ester and carboxylic acid, respectively. The different starting material structures between these cases and dissimilar conditions (solvents, droplet sizes, and spray distances) will contribute to this outcome. Additionally, the conversion of the ester to its corresponding acid via hydrolysis might also play a role in this process.
The most frequently suggested oxidizing agent in microdroplet-driven spontaneous oxidation is hydrogen peroxide/hydroxyl radical. Ongoing discussions continue regarding the production and yield of these species. In 2019, Zare and colleagues first reported the spontaneous generation of hydrogen peroxide in aqueous microdroplets produced by sonic spray (no applied electrical potential), with an observed concentration of H2O2 of 30 μM.13 Subsequently, they measured H2O2 levels in microdroplets formed by water vapor condensation, reporting concentrations in the range of 50–100 μM.77 However, these findings have been challenged by Mishra et al., who quantified H2O2 in water droplets and reported no detection of H2O2 in condensation microdroplets, with only 1 μM of H2O2 detected in microdroplets generated by sonication.78 This same research group later explored the impact of the surrounding air, suggesting that O3 in the air serves as the source of H2O2, rather than water itself, with the function of the water interface being to aid in mass transfer (note that in this study, control experiments were performed, showing no detectable H2O2 generation without O3).79 In response to this argument, Zare et al. re-evaluated the quantity of H2O2 generated in aqueous microdroplets in the absence of O3 (finding it to be less than 3 ppb), revealing the potential production of 0.3 to 1.5 μM of H2O2.80 Separately, Nguyen and colleagues proposed that ultrasonic cavitation is responsible for H2O2 generation, with the sonication process being involved in microdroplet formation.81
It is important to highlight two crucial considerations: (i) microdroplet reactions commonly involve solvent evaporation, droplet fission, ion ejection, and/or impurity adsorption, making the quantification of reactive species within microdroplets under ambient conditions a formidable practical challenge. (ii) The question of whether hydrogen peroxide is generated and to what extent microdroplets can undergo spontaneous oxidation reactions are interrelated yet distinct inquiries, given the likely existence of other oxidants. Given the intricate nature of identifying authentic oxidants, meticulous control experiments are necessary, although regrettably, they have been conducted in only a limited number of studies.13, 14, 16, 60, 62, 63, 67
Adding H2O2 into microdroplets is a simple way of testing evidence for H2O2 as the primary oxidant in spontaneous oxidations. However, only two studies have demonstrated an increase in oxidation yield with H2O2 addition, as observed in reactions 1.1 to 1.3.13, 63 Conversely, several studies have reported no change in oxidation yield when H2O2 is added, as evidenced in reactions 1.8, 1.11, 1.12, and 1.13.14, 16, 62, 67 Instead, alternative oxidants are suggested in the latter studies and supported by additional experimental evidence. In the oxidation of sulfones and sulfonamides (reactions 1.8 and 1.10), water radical cations were externally generated and added to the reaction system, and shown to result in increased conversion (Figure 4a).60, 62 In the examination of lipid oxidation, oxygen isotopic labeling experiments utilizing H2O18 revealed that the oxygen originates from the surrounding air (here, oxygen atom is the proposed oxidant), rather than from water itself, since 18O was not inserted into lipids when H218O was used as solvent (Figure 4b).16 More interestingly, even though hydrogen peroxide was found incapable of oxidizing these substrates, its production was still detected.16, 67

Representative control experiments to investigate oxidants for spontaneous oxidation in microdroplets. (a) Sulfone oxidation after addition of water radical cation to the reaction solution. Reproduced with permission.62 Copyright 2022 American Society for Mass Spectrometry. Published by American Chemical Society. (b) Lipid oxidation by O atom. Reproduced with permission.16 Copyright 2023 Royal Society of Chemistry.
Variations in reaction conditions also hold significance. We observe that in pure water droplets, H2O2/⋅OH is often suggested as the primary oxidant, while in organic droplets containing traces of water, water radical cation is proposed. Considering that in the solution phase, water radical cations can react with water molecules, particularly in water-rich environments, to generate hydroxyl radicals (eq. 3), we propose that water radical cations play a central role in all cases and serve as the source of H2O2/⋅OH. It is worth noting that this assumption harmonizes with the observation of decreased oxidation yields when additional water was added in sulfone or phosphonate oxidation (where water radical cation decays to H2O2/⋅OH, incapable of oxidizing these substrates),14, 62 and aligns with the outcomes of oxygen isotope experiments in lipid oxidation.16 As mentioned earlier, spontaneous reduction can coexist with oxidation, and O2 has been demonstrated to be captured and reduced to O2− (m/z 32) by either electrons or hydrated electrons (viz. water radical anions).8, 14 The resulting O2− also functions as an oxidant, facilitating the incorporation of oxygen from the surrounding air into substrates.
3.4 Mechanistic Overview of Spontaneous Oxidation in Microdroplets Driven by Water Radical Cation
The strong interfacial field is considered to be the driving force behind spontaneous oxidation and reduction in microdroplets. While direct evidence supporting the idea that the substrate (M) is oxidized by the electric field is lacking, this possibility cannot be dismissed (Figure 5a). As previously proposed, the electric field can facilitate the separation of charged water molecules, resulting in the formation of water radical cation and anion pairs (Figure 5b). In water-poor environments, such as at the droplet surface or in organic solvent droplets containing traces of water, the generated water radical cation may directly oxidize the substrate M, yielding the product (M=O or M+⋅). Conversely, in water-rich conditions, for instance, in the core of water droplets, the water radical cation would have a short lifetime and rapidly react with water, liberating hydroxyl radicals and hydrogen peroxide. This process may lead to the oxidation of M (if M has a low enough oxidation potential to be oxidized by H2O2/⋅OH) (Figure 5c) or the termination of the whole process. The latter scenario results in the observation of H2O2 production but an unchanged degree of oxidation when external H2O2 is added. Additionally, species in the surrounding gas, such as O2, may also participate in spontaneous oxidation, relying on the reduction of O2 to superoxide by the water radical anion liberated from the radical pair (Figure 5d). This general mechanism provides a rational explanation for all the observed experimental outcomes, highlighting the water radical cation/anion pair as the authentic chemical source for spontaneous redox chemistry in microdroplets.
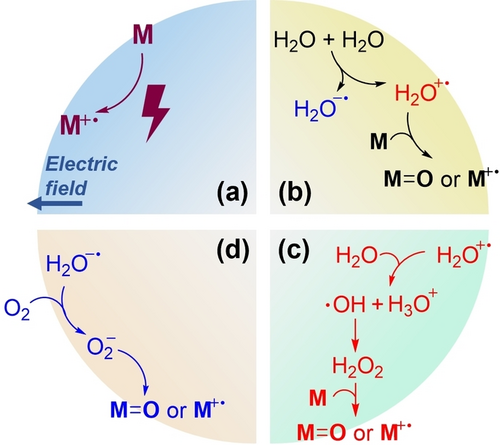
Overview of the mechanism for spontaneous oxidation in microdroplets driven by the water radical cation/anion pair, as facilitated by the strong interfacial electric field. (a) Direct oxidation by the interfacial electric field by electron detachment of substrate M. (b) Oxidation driven by water radical cation (free from water radical pair) in water-poor environment. (c) Oxidation driven by H2O2/⋅OH, produced from water radical cation in water-rich environment. (d) Oxidation by to O2 in air, where O2− is generated by reduction of O2 by water radical anion.
4 Future Directions
Looking ahead, there are several paths to explore. (i) Further mechanistic study: It has become evident from previous studies that several factors, including the amount of water in microdroplets, the ease of oxidation of substrates, the location of reactions (surface versus core), and the gaseous environment, can profoundly influence the nature of oxidation processes. Therefore, meticulous mechanistic investigations are warranted. (ii) Better control of competitive oxidation processes: It has been observed that for the same compounds or functional groups under similar droplet conditions, different products may arise. For instance, amines and benzoic acids can be oxidized to α-hydroxyl amines and phenols, respectively (as seen in reactions 1.5 and 1.7 in Table 1), but they have also been reported to undergo oxidative coupling (reaction 2.2 in Table 1). Investigating how to manipulate these competitive oxidation reactions is a compelling topic. (iii) Scaling up of spontaneous oxidations. Water radical cation, derived from water, presents an efficient and green oxidant. However, the limited scale of microdroplet reactions, typically products occur at low nanogram levels, constrains their practical application. Therefore, advancing the abundant generation and preservation of water radical cations and scaling up spontaneous oxidation processes could open new avenues for synthesis as well as for degradation of chemically inert molecules and materials.
Acknowledgments
The authors acknowledge financial support from the Multi-University Research Initiative (MURI) of the Air Force Office of Scientific Research (FA9550-21-1-0170) via Stanford University (sub-award 62741613-204669). We thank Dylan Holden for valuable discussions.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Biographical Information
R. Graham Cooks, Distinguished Professor at Purdue University and Fellow, National Academy of Sciences and National Academy of Inventors, is a recipient of the Dreyfus Prize. He has served as major professor to 156 Ph.D. students. Research interests include 1) ambient ionization and tandem M.S. analysis of complex mixtures, 2) mass spectrometry in cancer diagnostics and 3) high-throughput synthesis and analysis techniques, including drug discovery based on accelerated reactions in microdroplets.
Biographical Information
Lingqi Qiu received her B.S. and M.S. degrees in chemical biology from Peking University, specializing in organic synthesis and peptide synthesis. Later, she obtained her Ph.D. degree in Purdue University under the mentorship of Professor R. Graham Cooks. Lingqi's primary focus involves using mass spectrometry and chemical reactions to unravel complex chemical and biological processes.