Oxygen-Activated Boron Nitride for Selective Photocatalytic Coupling of Methanol to Ethylene Glycol
Abstract
The controllable photocatalytic C−C coupling of methanol to produce ethylene glycol (EG) is a highly desirable but challenging objective for replacing the current energy-intensive thermocatalytic process. Here, we develop a metal-free porous boron nitride catalyst that demonstrates exceptional selectivity in the photocatalytic production of EG from methanol under mild conditions. Comprehensive experiments and calculations are conducted to thoroughly investigate the reaction mechanism, revealing that the OB3 unit in the porous BN plays a critical role in the preferential activation of C−H bond in methanol to form ⋅CH2OH via a concerted proton-electron transfer mechanism. More prominent energy barriers are observed for the further dehydrogenation of the ⋅CH2OH intermediate on the OB3 unit, inhibiting the formation of some other by-products during the catalytic process. Additionally, a small downhill energy barrier for the coupling of ⋅CH2OH in the OB3 unit promotes the selective generation of EG. This study provides valuable insights into the underlying mechanisms and can serve as a guide for the design and optimization of photocatalysts for efficient and selective EG production under mild conditions.
Introduction
Ethylene glycol (EG) is of great significance in many aspects of our daily lives, serving as an essential component in numerous applications across various fields.1-3 The conventional approach for producing EG displays several obvious drawbacks, such as high energy consumption, harsh operation conditions, relatively low selectivity, and environmental pollution.1 It is imperative to develop an alternative synthetic route for EG production to address these problems. On the other hand, methanol has been extensively utilized for the production of multicarbon products via the C−C coupling.4, 5 Selective dehydrogenation and C−C coupling of methanol are highly expected to produce EG effectively with a greatly simplified synthesis route.6, 7 However, the synthesis route of EG faces significant challenges in three main aspects: (1) Selective dehydrogenation of sp3 α-C−H bonds to ⋅CH2OH is highly challenging because the methanol molecules are easy to lose hydrogen of O−H and C−H to form formaldehyde (HCHO).6 (2) Attaining precise C−C coupling is a key step to achieving high selectivity of EG. C−C coupling is commonly a kinetically slow and highly energy-absorbing process, remaining a fundamental bottleneck in the synthesis of EG.8, 9 (3) Preventing the further oxidation and dehydrogenation of intermediates is also difficult. The ⋅CH2OH intermediate is easily oxidized to form HCHO and formic acid, which will seriously affect the yield and selectivity of EG. Hence, designing and developing efficient and sustainable reaction methods and catalysts are of utmost importance.
Photocatalysis has emerged as a promising and energy efficient strategy for selective chemical transformations. Desired products can be selectively obtained by careful catalyst design and reaction conditions optimization.10 Photocatalytic selective dehydrogenation and coupling of methanol is undoubtedly an attractive approach for the production of EG, which is unattainable through traditional thermo-catalysis.7, 11 Currently, research in this field is still in its early stages and very limited catalysts have been developed for potential photocatalytic conversion of methanol to EG.7, 11-13 Besides, these reported catalysts primarily utilize metal-based materials with the addition of noble metal cocatalysts. Hence, there is a compelling need to design and develop efficient and cost-effective photocatalysts to realize the smooth transition from methanol to EG. Additionally, gaining an in-depth understanding of the reaction mechanism is crucial. These endeavors pose attractive and challenging targets for researchers in the field, aiming to advance the development of novel catalysts with improved performance for the photocatalytic conversion of methanol to EG.
Herein, we present the first report on metal-free porous boron nitride (BN) fibers for photocatalytic EG production. The BN fibers have been synthesized via an in situ high-temperature pyrolysis method and denoted as BNH. The BNH exhibits exceptional selectivity (97.6 %) in the selective photocatalytic dehydrogenative coupling of methanol to EG. To understand the underlying mechanism, a comprehensive set of characterizations and density functional theory (DFT) calculations have been performed. The results reveal that the incorporation of oxygen (O) atoms into the BN framework to form the OB3 structure, plays a crucial role in activating B atoms for the selective photocatalytic dehydrogenative coupling of methanol via a concerted proton-electron transfer (CPET) mechanism. Furthermore, the doping of O atoms effectively inhibits the recombination of electron-hole pairs, thereby enhancing the utilization efficiency of photogenerated carriers. These findings highlight the significance of O atom doping in BNH to form OB3 unit, as it not only enables the selective photocatalytic dehydrogenative coupling of methanol to EG but also improves the overall performance of the catalyst by promoting efficient charge transfer and utilization.
Results and Discussion
Field-emission scanning electron microscopy (FESEM) images (Figures 1a and b) and a transmission electron microscopy (TEM) image (Figure 1c) demonstrate the uniform size distribution of BNH with a significant aspect ratio and an average diameter of approximately 300 nm. Additionally, the rough surface and the presence of numerous light spots (Figure 1c) indicate the high porosity nature of the BNH. Furthermore, the high-resolution TEM (HRTEM) image (Figure 1d) reveals that the BNH is composed of many small units with a large interlayer distance of ≈0.345 nm, corresponding to the (002) lattice space. The selected area electron diffraction (SAED) pattern (Figure 1e) further shows the relatively low crystallinity of BNH. TEM elemental mapping (Figure 1f) demonstrates the O atoms are present and evenly distributed throughout the BNH. The XRD pattern of BNH (Figure 1g) shows a negatively shifted peak for (002) plane, which is probably attributed to the introduction of O atoms. Nitrogen adsorption/desorption isotherm and corresponding pore size distribution (Figure 1h) indicate that the BNH exhibits a type-IV isotherm with an H4 type broad hysteresis loop, suggesting the co-existence of micropores and mesopores in BNH.14, 15 These unique pore characteristics contribute to a significantly high surface area (719 m2 g−1) and high pore volume (0.43 cm3 g−1) (Table S1, Supporting Information), which is beneficial to the exposure of active sites.16 For comparison, commercially available boron nitride sheets (BNS) without O impurity have been utilized as reference material (Figures S1–4, Supporting Information).
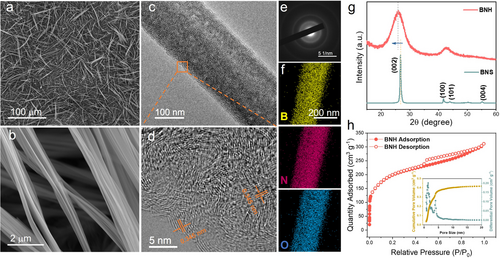
(a) FESEM image and (b) enlarged FESEM image of BNH. (c) TEM, (d) HRTEM images and (e) SAED pattern of BNH. (f) TEM elemental mapping of BNH. (g) XRD patterns of BNF and BNS. (h) N2 adsorption-desorption isotherms and pore distribution of BNH.
The X-ray absorption near edge structure (XANES) measurements provide insights into the chemical structure of BNH. The peak observed at 192.5 eV in the B 1s XANES spectrum (Figure 2a) is attributed to the transition of B 1s electrons to the π*(2pz) state, indicating a distinct signature of sp2 hybridization. The broad peak above 197 eV is assigned to the transition of B 1s electrons to the σ*(2px, y) state.17 Notably, compared with BNS, a new peak (A) at approximately 195 eV appears in the B 1s XANES spectrum of BNH. This peak is uniquely attributed to the transition of B 1s electrons to the unoccupied π*(2pz) state, which is associated with trigonal B bonded with O.17, 18 In the N K-edge spectra (Figure 2b), the peak at approximately 403 eV corresponds to the transition of N 1s electrons to the π*(2pz) state, and the peaks at approximately 410 eV and 417 eV correspond to the transition of N 1s electrons to the σ*(2px, y) state. In the O 1s XANES spectra (Figure 2c), two obvious new peaks at approximately 538.6 eV and 545.8 eV are observed in BNH, which are assigned to the transitions of O 1s electrons to the π*(OB3) and σ*(OB3) states, respectively. These results obviously indicate the existence of B−O bonds in BNH. In addition, the Fourier transform infrared (FTIR) spectroscopy of BNH (Figures 2d–f) shows distinct peaks at ≈3400 cm−1 and between 1000–1200 cm−1, which are assigned to OH-stretching and B−O stretching vibrations, respectively.19 Furthermore, the X-ray photoelectron spectroscopy (XPS) validates these observations by revealing a substantial presence of B−O bonds in BNH (Figure S5, Supporting Information).20 Additionally, the stronger electron paramagnetic resonance (EPR) signal (g=2.004) observed in BNH (Figure S6, Supporting Information) indicates the presence of a higher concentration of unpaired O in isolated conjugated OB3 unit,21 which corroborated the results mentioned above. The content of O element in BNH has also been measured by O elemental analyzer, which is significantly higher than that of BNS (Table S2, Supporting Information).
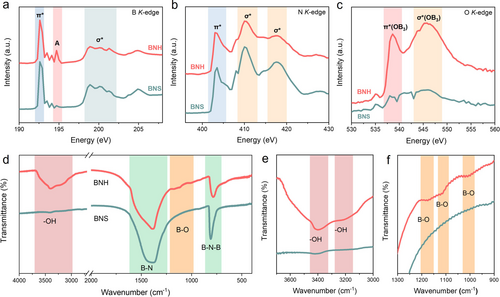
(a) The B K-edge, (b) the N K-edge and (c) the O K-edge XANES spectra of BNH and BNS. (d) FTIR spectra of BNH and BNS, enlarged FTIR spectra between (e) 3700–3000 cm−1 and (f) 1300–900 cm−1.
To investigate the impact of O atoms on the photocatalytic performance of selective dehydrogenation coupling of methanol to EG, photocatalytic experiments were carried out in a sealed quartz reactor filled with pure methanol under an argon atmosphere and irradiation of 500 W Xe-lamp. As shown in Figure 3a, the BNH exhibits a significantly enhanced EG production rate of 464.9 μmol g−1 h−1, and a high H2 generation rate of 496.1 μmol g−1 h−1, which are approximately 125 times and 14 times those of BNS (3.7 μmol g−1 h−1, 33.4 μmol g−1 h−1), respectively. Additionally, trace amounts of gaseous by-products such as monoxide carbon, methane, ethylene, and ethane are detected during the photocatalytic process (as shown in Figure 3a and Figure S7, Supporting Information). To evaluate the relative consumption of photogenerated holes and electrons, we calculated the n(e−)/n(h+) values by comparing the sum of n(H2) and n(CH4) to the sum of n(EG) and n(CO). The calculated n(e−)/n(h+) value is close to 1.04, indicating a nearly balanced consumption of photogenerated electrons and holes. The selectivity of EG based on the ratios of EG to the combined moles of H2 and CH4 has also been calculated. Remarkably, the BNH performs an impressive selectivity of 93.7 %. To more intuitively demonstrate the superior EG selectivity of BNH, we have also evaluated the selectivity based on a molar carbon basis, respectively (Figures S8 and S9, and Table S3, Supporting Information). The EG selectivity of BNH has been confirmed as 97.6 % which is comparable to the state-of-the-art reported catalysts (Table S4 and S5, Supporting Information).
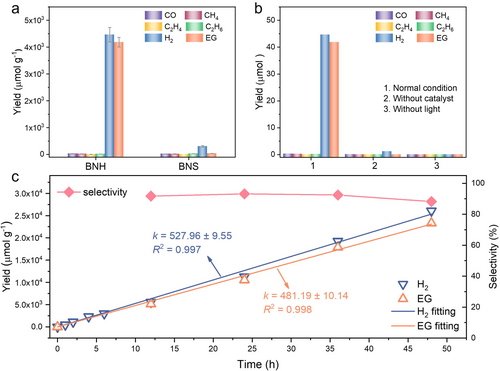
(a) Photocatalytic performance of BNH and BNS. Reaction conditions: 10 mg catalyst, 10 mL CH3OH solution, 500 W Xe lamp for 9 h. (b) Performances in different reaction conditions for 9 h. (c) Reaction stability test of BNH, corresponding fitting results, and EG selectivity at different reaction times, the EG selectivity was calculated on the ratio of n(EG) to n(H2) and n(CH4).
Controlled experiments have been conducted under various conditions (Figure 3b) to gain further insights into the methanol-to-EG process. No EG and any other by-products have been detected in the reaction systems without catalyst or in the dark condition, confirming that the photocatalytic conversion of methanol to EG is dependent on the presence of all required conditions. Long-term performance tests were carried out to evaluate the stability of the BNH catalyst. Remarkably, the BNH catalyst exhibits a sustained and steady rate over an extended reaction time, with the production of EG and H2 increasing linearly (Figure 3c). The slope values for the linear increase in EG (k=481.19±10.14) and H2 (k=527.96±9.55) indicate the consistent and reliable performance of the BNH catalyst over time. Notably, even after prolonged reaction periods, the BNH catalyst maintains a high selectivity for EG (≈90 %) (Figure 3c). This selectivity of BNH outperforms some of the previously reported materials employed in similar reaction systems.7 In addition, the TEM, XRD, FTIR, and XPS results show no obvious changes in BNH after reaction (Figures S10–13, Supporting Information), further illustrating the excellent reaction stability of BNH.
A series of experimental research and theoretical simulations were meticulously conducted to understand the reaction mechanism underlying the significantly enhanced performance of BNH. The surface chemical states and time-dependent electron density of BNH have been measured by in situ XPS measurements, which reveal conspicuous shifts in the peak positions of various elements upon irradiation. Specifically, in the B 1s spectra (Figure 4a), a negative shift indicates an augmented electron density surrounding the B atoms. Conversely, positive shifts in the O 1s spectra (Figure 4b) and N 1s spectra (Figure S14, Supporting Information) indicate a reduction in electron density around the O and N atoms.22 These alterations in binding energy furnish direct evidence elucidating the migration pathway of electrons from O and N to B atom under irradiation. It is known that the dynamics of photogenerated charge carriers play a critical role in determining the performance of catalysts.23, 24 Time-resolved photoluminescence (TRPL) spectroscopy measurements were conducted to evaluate the dynamics of charge carriers. As shown in Figure 4c, the average lifetime (τAve) of PL in BNH (3.3 ns) is significantly longer than that of BNS (1.08 ns), indicating the trap levels introduced via O-doping can effectively suppress the electron-hole recombination in BNH.25, 26 Furthermore, the lower impedance (Figure S15, Supporting Information) and higher photocurrent density (Figure S16, Supporting Information) observed in BNH suggest an enhancement in the separation and transfer of charge carriers.27 These results demonstrate that the O atoms in BNH promote charge separation and transfer and effectively suppress electron-hole recombination. In situ EPR spectroscopy measurements utilizing 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as a spin-trapping agent were also performed. Under irradiation, a spectrum attributed to a DMPO-CH2OH spin adduct is clearly observed in BNH (Figure 4d),11 while it could not be formed in the dark and is barely observed in BNS, indicating that the ⋅CH2OH is the major intermediate formed through the activation of C−H in methanol for EG production. In situ FTIR spectra of BNH show that two new peaks appeared at 1013.9 and 1052.2 cm−1, assigned to the coupling bands of the skeletal vibrational modes of C−O and C−C stretching vibration modes of EG (Figures S17 and S18, Supporting Information).28, 29 The increased intensities of these peaks with prolonged illumination duration provide further evidence for the generation of EG.
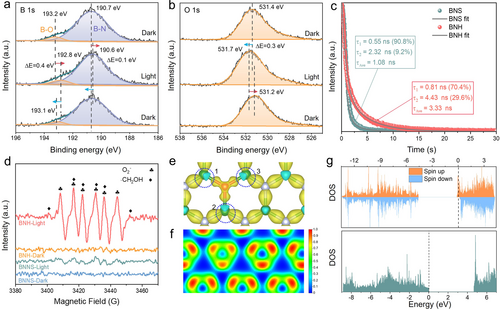
In situ high-resolution XPS spectra for (a) B 1s, (b) O 1s of BNH. (c) TRPL spectra and relative fitting results of BNH and BNS. (d) In situ EPR spectra for systems containing BNH and BNS catalyst in methanol aqueous solution in the presence of DMPO with or without light irradiation. (e) Charge density difference and (f) ELF contour (The blue and red regions were assigned to electron depletion and accumulation) of BNH model. (g) DOS of BNH (top) and BNS (bottom).
To explore the charge distribution in the BNH system, analyses involving charge density difference and electron localization function (ELF) contours were conducted in steady state (Figures 4e and f, Figure S19, Supporting Information). It is found the introduction of O atoms significantly affects the electronic structure of BNH, that the charge distribution is located almost completely around the O atom but a deficiency of electrons in the B atoms bonded to O atom, Furthermore, the substitution of O atoms engender an unpaired electron in comparison to N atoms, consequently leading to the existence of a free electron, which is more likely to migrate to B atoms during irradiation.17 As shown in Figure 4g, the density of state (DOS) of BNH with ON structure significantly differs from that of pure BN. In detail, the Fermi level of BNH drastically shifts into the conduction band in comparison with BNS, demonstrating the BNH has the nature of the n-type semiconductor. Additionally, the introduction of O dopant results in a narrower band gap of BNH compared to BNS, which is consistent with the experimentally determined band structures. (Figures S20–22, Supporting Information).
The optimized adsorption model of ⋅CH2OH on the catalysts, along with the corresponding charge density differences and relative ELF contour, are depicted in Figures 5a and b. Notably, a conspicuous chemisorption is observed between the B atom and ⋅CH2OH in BNH, indicating a strong and favorable interaction. In contrast, the interaction between BNS and ⋅CH2OH is considerably weaker, signifying a limited affinity between the B atoms in BNS and ⋅CH2OH. This observation indicates the pivotal role played by O-activated B atoms in facilitating the adsorption of ⋅CH2OH. Based on the experimental findings and DFT calculations, we proposed a possible reaction pathway as depicted in Figure 5c. Specifically, the C−H bonds of adsorbed CH3OH molecules are activated by the OB3 unit, resulting in the formation of ⋅CH2OH and ⋅H radicals. Subsequently, these radicals undergo a thermodynamically downhill coupling reaction to form EG and H2 molecules.
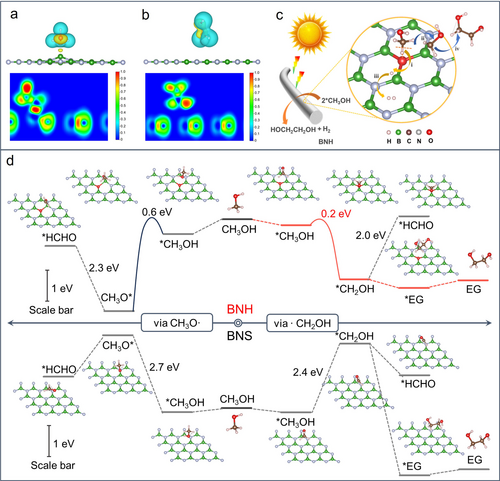
Optimized adsorption model and charge density differences and relative ELF contour of ⋅CH2OH chemisorbed on (a) BNH and (b) BNS. (c) Schematic illustration of BNH for photocatalytic synthesis of EG and H2 from methanol. (d) Reaction pathways for photocatalytic coupling of methanol to EG on BNH and BNS and the corresponding atomic configuration of BNH and BNS catalyst systems.
The transformation processes from methanol to either EG or formaldehyde on BNH and BNS catalysts have been thoroughly investigated by comprehensive calculations (Figure 5d). It reveals that the BNH with the OB3 unit exhibits much lower energy barriers for the dehydrogenation of C−H and O−H bonds in methanol compared to the BNS with NB3 structure, which is a crucial factor contributing to the enhanced performance of BNH. Importantly, DFT calculations also provide insights into the high selectivity of BNH towards EG production. The reaction barrier energy for the formation of the ⋅CH2OH intermediate (≈0.2 eV) has been confirmed, which is much lower than that of the CH3O⋅ intermediate (≈0.6 eV), indicating a preferential cleavage of the C−H bond in methanol to form ⋅CH2OH through a CPET process.7, 30 Moreover, the energy barriers for the conversion of CH3O* to HCHO* and *CH2OH to *HCHO are prohibitively high, effectively inhibiting the excessive oxidation of methanol to HCHO or other by-products. Furthermore, the generated intermediate ⋅CH2OH species are adsorbed onto the OB3 unit, where they undergo a thermodynamically favorable coupling reaction leading to the formation of EG. This coupling process is significantly more favorable than the formation of HCHO. Therefore, the BNH exhibits high yield and ultrahigh selectivity for EG production from methanol due to the combination of preferential C−H bond cleavage, suppression of excessive oxidation, and thermodynamically favorable coupling reactions within the OB3 unit.
Conclusion
In summary, the BNH catalyst, prepared via an in situ high-temperature pyrolysis method, exhibits an ultra-high selectivity for EG through the photocatalytic dehydrogenation coupling of methanol. The comprehensive experimental results confirm that the construction of OB3 units in BNH plays a crucial role in the EG production process, significantly influencing the electronic structure of the BNH and suppressing the recombination of electrons and holes, resulting in high efficiency and ultra-high selectivity of EG. The calculations further thoroughly reveal the reaction mechanism, showing that the OB3 unit in BNH shows a lower energy barrier for dehydrogenation of C−H bond in methanol compared to O−H bond, facilitating the generation of the important intermediate (⋅CH2OH) for EG production. Importantly, the OB3 unit also has a small energy barrier for coupling of ⋅CH2OH, and large energy barriers for further dehydrogenation of CH3O⋅ and ⋅CH2OH, which are the main reasons for significant promotion in the selectivity of EG. These findings provide valuable insights into the underlying mechanisms and can guide the design and optimization of catalysts for efficient and selective EG production under mild conditions.
Acknowledgments
J. Liang and Q. Song contributed equally to this work. This work was supported by Research Grants Council of the Hong Kong Special Administrative Region, China (C1009-17EF), National Natural Science Foundation of China (Grant No. 22178149), Natural Science Foundation of Jiangsu Province for Outstanding Youth Scientists (BK20211599). The authors thank Prof. Li Song from University of Science and Technology of China for the kind support of the XAF measurements.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




