Impact of the Core Chemistry of Self-Assembled Spherical Nucleic Acids on their In Vitro Fate
Abstract
Nucleic acid therapeutics (NATs), such as mRNA, small interfering RNA or antisense oligonucleotides are extremely efficient tools to modulate gene expression and tackle otherwise undruggable diseases. Spherical nucleic acids (SNAs) can efficiently deliver small NATs to cells while protecting their payload from nucleases, and have improved biodistribution and muted immune activation. Self-assembled SNAs have emerged as nanostructures made from a single DNA-polymer conjugate with similar favorable properties as well as small molecule encapsulation. However, because they maintain their structure by non-covalent interactions, they might suffer from disassembly in biologically relevant conditions, especially with regard to their interaction with serum proteins. Here, we report a systematic study of the factors that govern the fate of self-assembled SNAs. Varying the core chemistry and using stimuli-responsive disulfide crosslinking, we show that extracellular stability upon binding with serum proteins is important for recognition by membrane receptors, triggering cellular uptake. At the same time, intracellular dissociation is required for efficient therapeutic release. Disulfide-crosslinked SNAs combine these two properties and result in efficient and non-toxic unaided gene silencing therapeutics. We anticipate these investigations will help the translation of promising self-assembled structures towards in vivo gene silencing applications.
Introduction
Therapeutic nucleic acids, such as antisense oligonucleotides (ASOs), small-interfering RNAs (siRNAs), and mRNA have demonstrated significant efficacy for clinical use through their ability to modulate gene expression.1 In order to elicit an efficient response, these nucleic acid therapeutics (NATs) must not only withstand degradation by endogenous enzymes known as nucleases but must also be capable of reaching the desired organ and tissue where therapeutic action is required. Essential strategies have pioneered the clinical use of NATs, such as the development of chemically modified nucleic acids that are more resistant to degradation,2 or encapsulation of these therapeutics within larger nanocarriers3, 4 to shield them from enzymes and allow for a more targeted biodistribution.
Spherical nucleic acids (SNAs) have been recognized as prime candidates for nucleic acid delivery due to their enhanced nuclease resistance,5 cellular uptake,6 and improved biodistribution.7, 8 While conventional SNAs typically consist of a metallic-based core, alternative and more biocompatible9, 10 cores, such as liposomes or hydrophobic polymers, have been introduced to circumvent cellular and systemic toxicity. An important aspect that needs to be taken into consideration when choosing a suitable core is the stability of the SNA under physiological conditions, both extra- and intracellularly, and its effect on therapeutic activity.
Our laboratory has introduced self-assembled SNAs based on microphase separation of DNA-polymer conjugates: adapting the chemistry of DNA solid-phase synthesis, we incorporate hydrophobic monomers in a sequence-controlled manner within a nucleic acid sequence, resulting in DNA amphiphiles. In buffer solutions, these amphiphiles self-assemble into SNAs with low polydispersity.11 By strategically altering the chemical nature of the hydrophobic monomers within the amphiphile, it is possible to modulate the core's stability and overall morphology.12 In this work, we report a systematic investigation of the different conditions needed for self-assembled SNAs to be efficient gene silencing oligonucleotide carriers without relying on transfection agents. First, we designed SNAs with a tightly packed hydrophobic core, resulting in micellar structures that withstand denaturing conditions and efficiently encapsulate drug-like small molecules. We introduced disulfide moieties within the core to allow for dynamic covalent crosslinking, stabilizing the structure while ensuring intracellular dissociation by the reductive cellular environment. Such a strategy has been proven to largely improve stability of polyplex micelles for the delivery of plasmid DNA13-16 and siRNA,17-19 resulting in extended in vivo circulation and intracellular responsiveness. We studied the extracellular stability (Figure 1-I) of these different structures and how their interaction with serum proteins affects their cellular uptake (Figure 1-II). After cellular uptake, endosomal escape and therapeutic release is crucial for efficient gene knockdown (Figure 1-III). Introducing an oligonucleotide therapeutic as the SNA corona, we investigated the influence of the core chemistry on these parameters and on gene silencing ability (Figure 1-IV). Our investigations show that the SNA core must withstand extracellular dissociation for efficient cellular uptake, while simultaneously being able to dismantle once inside the cell, to release nucleic acids, so that the SNAs can subsequently elicit their intended gene silencing response. Through these studies, we identified an optimal SNA candidate with a disulfide-crosslinked core for efficient gene silencing activity with low toxicity.
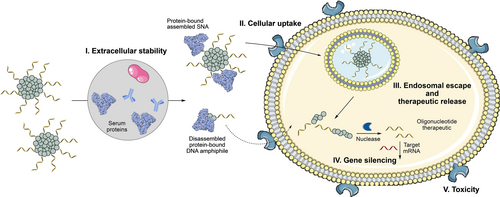
In physiological conditions, self-assembled SNAs face multiple obstacles: extracellular stability and possible disassembly by exposure to serum proteins (I) that impact their cellular uptake (II). This can be prevented by increased core hydrophobicity (C6, Figure 2) or core crosslinking (S6x, Figure 2, 3). Following internalization, however, the requirements are reversed: the nanostructure should be able to perform its biological function, i.e. escape the endosome and release the oligonucleotide therapeutic (III) as well as dissociating for gene silencing (IV) without inducing toxicity (V). Overall, these contradictory requirements are fulfilled by a disulfide core-crosslinked SNA (S6x, Figure 2, 3), which resists dissociation extracellularly, escapes the endosome and undergoes reduction-induced dissociation intracellularly.
Results and Discussion
Design and Characterization of Core-Minimal SNAs
Our group has previously shown the importance of structure and sequence of the hydrophobic building blocks, in DNA amphiphiles that self-assembled into SNAs. For example, adding branched rather than linear hydrophobic units in strategic locations improved the SNA gene silencing activity.12 We proposed that this is likely due to an alternative mode of core packing involved in the self-assembly of branched hydrophobic units compared to their linear variants,20 which reduces the need for high concentrations of divalent cations to remain assembled and improves overall stability (Figure S13A). We therefore first opted to develop SNAs with a new branched hydrophobic core, with an alkyl chain attached to a 1,2-diol moiety, to investigate their self-assembly and stability (Figure 2).
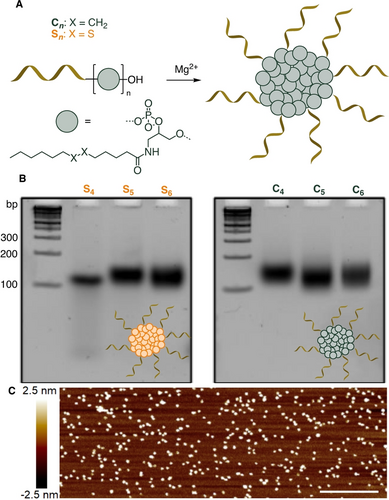
A) Design and assembly of core-minimal SNA. B) Native PAGE (8 %) of SNAs S4–6 and C4–6 that show low mobility (>100 bp for 24-mer strands) bands corresponding to self-assembled products. C) Representative AFM image of C5 deposited on mica surface (see Figure S2). Scale bar=250 nm.
We synthesized two new branched phosphoramidites for solid-phase oligonucleotide synthesis: a disulfide-containing moiety to create a bio-reducible core that would induce the intracellular disassembly of our SNAs in reductive cellular environment, as well as a control variant containing only an alkyl chain (Figure 2A, Scheme S1). These phosphoramidites were incorporated by oligonucleotide automated synthesis using a DNA synthesizer, with 4 to 6 repeats to create DNA amphiphiles S4–6 and C4–6, containing disulfide or alkyl chains, respectively (Figure 2A). All synthesized amphiphiles (Table S1) were found to self-assemble into SNAs of well-defined size and shape as well as low polydispersity, as observed by low mobility bands (>100 bp for 24-mer strands) on native polyacrylamide gel electrophoresis (PAGE, Figure 2B), agarose gel electrophoresis (AGE, Figure S10), by atomic force microscopy (AFM, Figure 2C, Figure S2), and dynamic light scattering (DLS, Table S3). Interestingly, contrary to SNAs made from linear hydrophobic blocks, C6 and S6 maintained their assembled structures in Mg2+-free conditions (Figure S3, S4). C6 remained assembled even under the denaturing PAGE conditions, highlighting its increased stability. On the other hand, the slightly less hydrophobic amphiphile S6 moves as a non-assembled single strand amphiphile in denaturing conditions, but also assembles without the need for Mg2+ cations in native conditions (Figure S3).
To further strengthen the core of disulfide-containing SNAs and provide additional stability in cell culture medium, we sought to take advantage of the disulfide moieties in the S6 SNA to covalently crosslink the hydrophobic core, moving from non-covalent to covalent interactions to prevent possible disassembly. Two methods were used to perform this crosslinking. First, we set out to partially reduce the disulfides in S6 using TCEP (tris(2-carboxyethyl)phosphine) as a reducing agent before assembly (Figure 3A). TCEP partial reduction results in a mixture of thiols and disulfides that should exchange at basic pH during the annealing process to crosslink the different strands together (Figure 3A, Scheme S2A). Denaturing PAGE shows the presence of multiple lower mobility bands, indicating that crosslinking occurred to give oligomers (Figure 3D). Alternatively, the assembled S6 SNA was incubated with an excess of a tetrathiol crosslinker 1 (Figure 3C, Scheme S2B),21 which yielded more efficient crosslinking as observed by denaturing PAGE (Figure 3D). We confirmed that these three structures remained assembled as micelles by native AGE (Figure 3B) and AFM (Figure S6), and were bioreducible, dissociating upon incubation with reducing agent (Figure S8). The excess of crosslinker was then removed by dialysis (Figure S5).
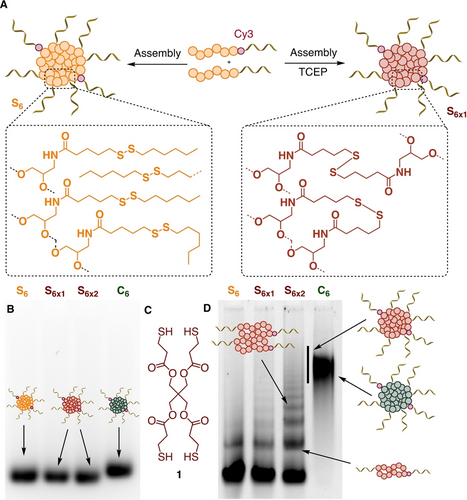
A) Schematic representation of the crosslinking of disulfides in the SNA core. B) Native AGE gel (2 %, Cy3 channel) of non-crosslinked and crosslinked Cy3-labelled SNAs showing similar mobilities (S6x1=TCEP-mediated crosslinking, S6x2=tetrathiol 1-mediated crosslinking). C) Structure of tetrathiol crosslinker 1 used to crosslink S6 into S6x2. D) Denaturing PAGE gel (6 %, Cy3 channel) of non-crosslinked and crosslinked SNAs showing the appearance of oligomers of S6 upon crosslinking.
Self-assembled micelles can encapsulate small molecules within their hydrophobic core, and disulfide crosslinking of the core has been shown to result in on-demand drug release.22, 23 Disulfide-crosslinked DNA nanostructures, especially hydrogels24 and large DNA origami,25 have also been reported to respond to endogenous glutathione. Previous experiments showed efficient encapsulation during SNA self-assembly of the fluorogenic dye Nile Red, and of chemosensitizers within self-assembled SNAs made of twelve linear hydrophobic dodecane chains ((C12)12, Figure S13A). We noted that both C6 and S6 could also encapsulate Nile Red in their hydrophobic core, with higher fluorescence emission intensity observed for C6 as compared to (C12)12 (Figure S13B). Addition of the hydrophobic cargo to pre-formed C6, S6, S6x1 and S6x2 led to overall lower encapsulation (Figure S13C). Interestingly, upon incubation with the reducing agent dithiothreitol (DTT), disulfide containing SNAs showed a decrease of fluorescence, consistent with their disassembly (Figure S13C).
Interaction with Serum Proteins
We then sought to investigate the fate of these SNAs in physiologically relevant conditions, especially with regard to interaction with serum proteins (Figure 1-I), as these tend to dissociate self-assembled structures.26 To evaluate this, we incubated S4–6 and C4–6, containing a phosphorothioated ASO corona, in media containing 10 % of fetal bovine serum (FBS). We observed that the initially self-assembled SNA band shifts to a lower mobility band on native AGE (Figure 4A, “Ser”, Figure S14). This indicates that our constructs interact with components of serum to some extent. Repeating the same experiment with albumin-depleted serum (“Ser-”), we observed that the SNAs maintained the same mobility, suggesting that albumin is the main protein involved in this interaction. This was confirmed by incubating SNAs with human serum albumin (HSA), which led to a similar shift in mobility as what was observed in serum. After incubating these HSA-dosed samples with proteinase K to degrade the protein, we observed the re-emergence of the initial SNA band (Figure 4A, “HSA-”), which confirms the interaction of HSA with our structures. This is in line with the known micromolar affinity of HSA towards phosphorothioate PS-DNA, in addition to its interaction with the polymer alkyl chains.27
While this confirmed that albumin indeed interacts with our constructs, we wanted to determine whether the SNAs maintained their integral structures upon interaction with HSA, or whether the protein induced SNA dissociation. To study the fate of these self-assembled SNAs with albumin in further detail, we turned to size exclusion chromatography (SEC). C6, S6 and S6x SNAs were incubated with 10 % of FBS to mimic cell incubation conditions (Figure 4C). In the absence of FBS, all samples have a retention time between 6 and 7 min, corresponding to the assembled structure (Figure 4B*) with trends in sizes matching those observed by AGE and AFM (Table S2, Figure S2, S6). In both crosslinked S6x, a small portion of strands do not self-assemble, likely corresponding to over-reduced amphiphiles that have lost their hexylthiol chains. After incubation in FBS, peaks corresponding to S6 disappear and only peaks with albumin retention time (Figure 4C++) are observed, consistent with total disassembly of the SNA. Noticeably, these peaks shift to slightly lower retention time because of a slight increase of size due to the binding of the DNA amphiphile to albumin. On the other hand, C6 retains a broad peak at lower retention times (5.8 vs 6.5 min before incubation) that likely corresponds to assembled SNAs coated with a protein corona, without disassembling (Figure 4C+). Both crosslinked S6x also maintain a similar peak of protein-coated SNA in FBS, albeit with lower intensities as compared to C6, suggesting partial disassembly. This trend of stability toward dissociation matches the observation that C6 is more stable than S6 in denaturing conditions (Figure S4). Thus, the non-crosslinked S6 SNA fully degrades to its albumin-bound DNA amphiphile in serum, while more intact C6 and crosslinked S6x SNAs remain in these conditions.
Cellular Uptake of Spherical Nucleic Acids
After confirming higher stability in contact with HSA upon crosslinking, we studied the influence of this parameter on cellular uptake (Figure 1-II). The presence of serum proteins has been shown to greatly reduce the uptake of gold nanoparticle SNAs by forming a protein corona shielding the oligonucleotides from their receptors.28 We thus expect the different extracellular stability profiles of our SNAs to influence their cellular uptake.
We incubated HeLa cells with Cy3-labelled non-crosslinked S6, crosslinked S6x1 and S6x2, and C6, for 1, 4, and 24 h at 37 °C and measured cellular uptake by flow cytometry (Figure 5A) after washing the cells to remove extracellular and membrane-bound DNA. In these structures, the DNA corona is fully phosphorothioated (PS), and the Cy3-label is at the interface between the DNA and hydrophobic core to avoid its cleavage by nucleases. At every point, C6 was the most internalized construct. While the disulfide SNAs showed comparable uptake profiles at 1 h of incubation, S6x2 showed 40 % higher uptake than S6 and S6x1 at 4 h incubation. Finally, after 24 h, all disulfide SNAs showed similar uptake. We have shown earlier that C6 and S6x2 are the most stable to albumin-mediated dissociation (Figure 4C). This suggests a correlation between SNA extracellular stability and cellular uptake, with stable constructs more readily taken up than albumin-bound, disassembled structures.
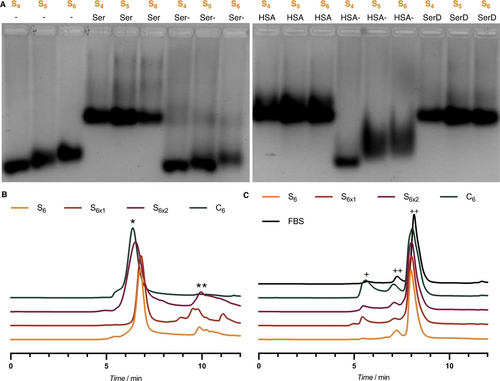
A) Electrophoretic Mobility Shift Assay (EMSA, 2 % native AGE) of S4, S5 and S6 SNAs interaction with serum proteins. Ser=Serum; Ser-=Albumin-depleted serum; HSA=Human Serum Albumin; HSA-=HSA degraded by PK treatment; SerD: Diluted Serum. B) Normalized size exclusion chromatograms of S6, S6x1, S6x2 and C6, *=SNA; **=non-assembled DNA amphiphile. C) Normalized size exclusion chromatograms of S6, S6x1, S6x2 and C6 after 1 h incubation at 37 °C in 10 % FBS, +=SNA with protein corona; ++=Albumin. Elution buffer: 1× TAMg.
After 24 h of incubation, the cellular uptake was similar for all disulfide SNAs, which we suggest may be due to prolonged incubation in serum resulting in the de-crosslinking of the disulfide core in S6x. We ran a control experiment incubating S6x1 with HSA for 2 h and observed a partial loss of crosslinking (Figure S9). This may be due to interactions with the free cysteine residue and disulfides in the HSA proteins bound to the construct, resulting in a partial de-crosslinking, until the structures finally exhibit just as much cellular uptake as the already dissociated S6. These experiments confirm that crosslinking and enhanced stability of the SNA core have a positive impact on the cellular uptake of these structures under biological conditions, as they remain in their assembled state.
Next, we investigated the uptake pathways that were engaged during this process. We co-incubated HeLa cells with SNAs and an inhibitor of clathrin-mediated endocytosis (sucrose29) or a non-selective inhibitor of scavenger receptors (polyinosinic acid30) for 4 h. In this study, we observed that the uptake of all SNAs tested was partially inhibited by polyinosinic acid (Figure 5B), suggesting their uptake by interaction with scavenger receptors.6 Upon incubation with sucrose, C6 and both crosslinked S6x showed a similar decrease in cellular uptake while S6 maintained the same uptake levels (Figure 5B). This suggests that intact SNAs C6 and S6x might engage additional pathways toward endocytosis compared to disassembled S6, possibly explaining their higher uptake. Interestingly, albumin-bound structures have been shown to be taken up through recognition by scavenger receptors,31 but not through clathrin-mediated pathways.32 This is consistent with S6 disassembling into its albumin-bound individual strands and being taken up by scavenger receptors, but not engaging additional clathrin-mediated pathways. On the other hand, several scavenger receptors mediate cellular uptake by both clathrin-dependent and independent processes,33 which could be responsible for the uptake of C6 and S6x. These experiments show that SNAs are taken up by scavenger receptors, with C6 and both crosslinked S6x showing additional clathrin-mediated cellular uptake pathways, while engaging similar levels of clathrin-independent uptake pathways as compared to S6. This shows that core stability (C6) or core crosslinking (S6x2) results in more assembled SNAs in serum and greater cellular uptake.
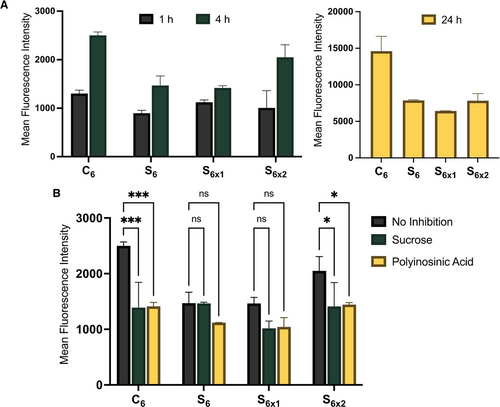
A) Measured fluorescence intensity in HeLa cells after incubation with 1 μM of 10 % Cy3-doped C6, S6 and S6x (S6x1=TCEP, S6x2=tetrathiol 1) for 1, 4 or 24 h to demonstrate cellular uptake of different constructs with time. 1 h: ns difference between C6, S6, S6x1 and S6x2; 4 h: C6 ** vs S6 and S6x1, ns vs S6x2, ns difference between S6 and S6x1; 24 h: C6 **** vs all, ns difference between S6, S6x1 and S6x2. B) Measured fluorescence intensity in HeLa cells after incubation with 1 μM of 10 % Cy3-doped C6, S6 and S6x for 4 h without inhibitor or co-incubated with sucrose (150 mM) or polyinosinic acid (500 μg/mL). The Cy3 unit was placed at the interface between the DNA corona and the core to avoid nuclease-induced dissociation. Error bars represent SD of duplicate experiments for each sample. ****: p<0.0001; ***: p<0.0002; **: p<0.0021; *: p<0.033; ns: p>0.123.
Transfected Gene Silencing Activity
After studying how extracellular stability plays a role in the cellular uptake of self-assembled SNAs, we sought to investigate the parameters needed for their gene silencing activity once they get internalized into cells. To do so, we first opted to transfect the structures with the help of a transfection agent in Opti-MEMTM culture medium, thus circumventing the need for extracellular stability and receptor-mediated cellular uptake, focusing solely on discrepancies due to different intracellular fates of the SNAs.
The oligonucleotide sequences used as the corona in these SNAs were fully phosphorothioated ASO, protected against possible degradation by serum nucleases and designed to silence luciferase expression in HeLa X1/5 cells. We first measured the gene silencing activity of all SNAs S4–6 and C4–6 when transfected with LipofectamineTM 3000 (Figure 6A). We found that all SNAs were non-toxic at these concentrations, where the unmodified PS-ASO suffers from poor cell viability (Figure S24A). Within the C series, the smaller the hydrophobic core, the higher the gene silencing activity. We hypothesize that cytosolic disassembly of the SNA is needed for high gene silencing activity. By being held together by stronger hydrophobic effect as compared to the less hydrophobic C4, SNAs C5 and C6 likely stay assembled inside cells, impairing the interaction of the ASO with luciferase mRNA and resulting in lower activity. On the other hand, we found that SNAs with a bioreducible disulfide core S4–6 resulted, in all cases, in higher gene silencing activity, with little impact of the number of hydrophobic modifications. We reason that the bioreducible SNAs can fall apart in the reducing cellular environment, cleaving off the 1-hexanethiol chains and drastically reducing the amphiphiles’ hydrophobicity, resulting in disassembly (Figure S24B). These experiments suggest that intracellular core dissociation is beneficial for gene silencing activity. Since C6 and S6 showed the largest differences in this assay, the focus was kept on their direct comparison.
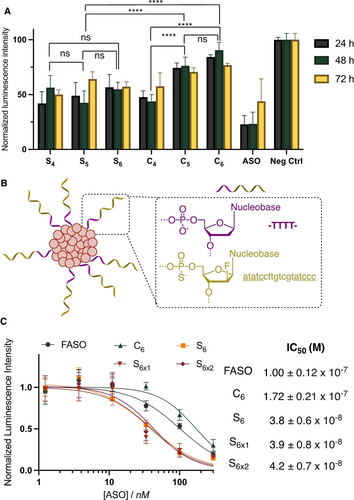
A) Firefly luciferase knockdown activity after incubation for 24 h in HeLa cells with 500 nM of SNA transfected with LipofectamineTM 3000. B) Schematic representation of SNA including a polythymidine spacer in addition to a gapmer FASO sequence to improve silencing. Underlined=2′-FANA. C) Firefly luciferase knockdown activity after incubation for 24 h in HeLa cells with varying concentrations of FASO, S6, S6x1, S6x2 and C6 transfected with LipofectamineTM 3000. This data was used to extrapolate the IC50 concentrations of the different constructs. Error bars represent SD of six replicates for each sample. ****: p<0.0001; ***: p<0.0002; **: p<0.0021; *: p<0.033; ns: p>0.123.
Previous work from our group showed that the incorporation of 2′-fluoroarabinose nucleic acid (FANA) modifications on the antisense portion of SNAs improved gene silencing activity. We thus used this modification to design potent gene silencing SNAs. The hydrophobic core was kept untouched with either S6 or C6, and a previously designed FANA gapmer34 that was also fully phosphorothioated was used as the ASO (FASO, Table S1). An endonuclease-cleavable phosphate tetrathymidine spacer was introduced at the interface, as we previously showed that its cleavage and release of the ASO from the SNA core led to higher gene silencing activity (Figure 6B).34 We verified that the introduction of this spacer did not lead to instability in serum (Figure S18), as was originally reported for this construct with a different core.
To study the impact of the core design on intracellular disassembly and therapeutic release, we compared the efficiency of luciferase knockdown of C6, S6 and both S6x upon transfection by LipofectamineTM. As the transfection agent facilitates cellular uptake and endosomal escape, this experiment mainly compares the ability of our constructs to dissociate and release therapeutics intracellularly, as well as the interaction between the structure and the transfection agent. After 24 h of incubation, the structures showed different levels of gene silencing (Figure 6C): while incubation with C6 led to a higher IC50 than the non-modified FASO (172 vs 100 nM), S6 (crosslinked or not) showed an order of magnitude higher activity, with an IC50 around 40 nM regardless of the crosslinking. Together with the initial transfection experiments (Figure 6A), this data shows that the bioreducible disulfide core results in higher activity, likely due to the dissociation of the SNA in reductive conditions allowing for a more significant release of the therapeutic. Conversely, the activity of C6 is lower under these conditions, presumably because its robust hydrophobic core does not dissociate as readily. When considering the interaction of the structure with the transfection agent, a desirable outcome is the strong binding of the structure to the agent extracellularly to increase the amount transported into the cell, but a weaker interaction intracellularly, so the transfection agent can release the therapeutic. For the S6 series, these two requirements are met: extracellularly, the interaction of the transfection agent with the alkyl chains in the structure strengthens the binding. Intracellularly, the ability of the S6 core to be reduced and release its alkyl chains weakens the interaction with the transfection agent. This can explain the significantly higher activity of S6 as compared to unconjugated FASO, as well as the lower activity of the non-reducible C6.35 Thus, while the extracellular stability of C6 results in greater cellular uptake, its decreased ability to dissociate intracellularly lowers its gene transfection agent-induced silencing efficiency. On the other hand, the combination of endonuclease-mediated cleavage of the tetrathymidine spacer and reduction of the SNA core for S6 and S6x, allows for more pronounced gene silencing to take place.
Endosomal Escape and Therapeutic Release
Endosomal escape is key for the efficiency of nucleic acid therapeutics and remains the major bottleneck in their development. A key to the success of mRNA lipid nanoparticle vaccines has been the introduction of ionizable lipids in their composition to disrupt the endosomal membrane and prevent entrapment.36 On the other hand, siRNA-GalNAc conjugates have been shown to mostly remain in the endolysosomal network, resulting in the slow release of siRNA to the cytosol and extremely prolonged efficiencies, up to 6 months after injection in vivo.37 To study the membrane lysis activity of the different constructs, we turned to an established assay measuring the ex vivo hemolysis of red blood cells (RBC) at different pH to mimic the different stages of the endolysosomal network: 7.4 (extracellular environment), 6.8 (early endosomes) and 5.5 (late endosomes).38 An ideal system would only result in membrane lysis at acidic pH, corresponding to destabilization of the endosomal membrane and resulting in escape. Upon incubation with the non-amphiphilic FASO, S6 or C6, minimal hemolysis (<10 %) was observed, indicating marginal membrane lysis is occurring at any stage of the endocytic process (Figure 7A). S6x1 and S6x2 showed a similarly low hemolytic behavior, which appeared to be occurring in a pH-responsive manner, with membrane lysis occurring more prominently under acidic conditions, but this trend was not significant. These results are to be expected since the SNA core chemistry was not designed to contain pH-responsive moieties, such as ionizable lipids, that would enable endosomal escape through membrane lysis at acidic pH. This would suggest maintenance of our constructs in the endolysosomal network, resulting in more prolonged release of therapeutic into the cytosol rather than rapid release through immediate endosomal lysis.
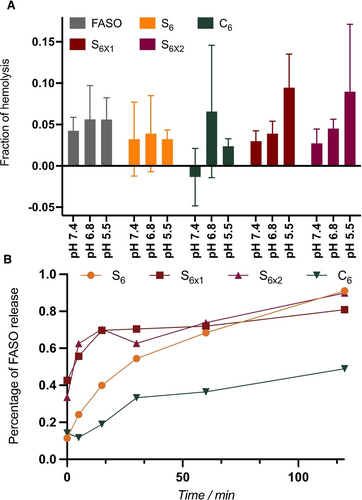
A) Percentage of lysis of red blood cells after 1 h incubation with the SNAs at different pH, corresponding to membrane lysis. Error bars represent SD of six replicates per sample. B) Percentage of FASO release from the SNAs after incubation with DNAse I as a function of incubation time, quantified by PAGE.
To reach high silencing activities, however, only the FASO portion of the SNAs needs to reach its cytosolic target unhindered by the attached hydrophobic units, hence the introduction of an endonuclease-cleavable spacer (Figure 6B) to cleave the FASO from the SNA core.34 The rate of this cleavage after incubation with DNAse I was analyzed by PAGE (Figure 7B, S15–S17) and showed that C6 is cleaved at a lower rate than all S6 SNAs, crosslinked or not. After the action of endonucleases in the endolysosomal network,39, 40 several pathways of endosomal escape could occur for the FASO, as described in the literature.41, 42 The intracellular trafficking of phosphorothioated oligonucleotides has been widely studied and explains their higher activity as compared to non-modified ASOs.43-45 From these experiments, S6 and S6x SNAs show the most promise for unaided gene silencing, as they are able to dissociate intracellularly due to their bioreducible core and release the FASO therapeutic the most efficiently.
Unaided Gene Silencing
With these results, we can determine rules that govern the in vitro fate of the SNAs studied here. Whereas C6 is uptaken the most, as a result of its greater extracellular stability, its low endosomal escape and slower therapeutic release combined with low transfected activity should result in low unaided gene silencing. Crosslinking of S6 SNAs was showed to be beneficial for their cellular uptake while also benefiting from higher transfected gene-silencing activity, likely due to their bioreducible nature, which should result in the best unaided gene silencing in the series studied here. We therefore compared these predictions with the unaided (gymnotic) gene knockdown in the absence of transfection agents. This will provide guidelines as to the importance of all the different factors in the overall performance of the constructs: extracellular stability, cellular uptake, intracellular dissociation, endosomal escape and therapeutic release.
Transfection agent-free gene silencing experiments were performed by incubating luciferase-expressing HeLa cells with 1 μM of SNA solutions for 24, 48, and 72 h and measuring the residual luciferase activity (Figure 8A). We found that although C6 was the worst performer and had the highest IC50 when transfected with LipofectamineTM, it consistently silenced around 50 % of luciferase activity at all time points over 72 h, only slightly losing activity over time. On the other hand, despite its lower IC50 upon transfection, S6 did not perform as well compared to C6. This suggests that the much higher cellular uptake of C6 as compared to S6 compensates for its lower activity once inside the cell. S6x1 behaved similarly to S6, consistent with their similar cellular uptake and transfected silencing profiles. However, C6 exhibits cytotoxicity after prolonged incubation time (Figure 8B). This is possibly due to its non-reducible nature which might result in undesirable membrane interactions over long periods of time. Despite being low, the activity of S6 gradually improves slightly over time without any cytotoxicity effect, possibly benefiting from a gradual reduction of the core and improved therapeutic release.
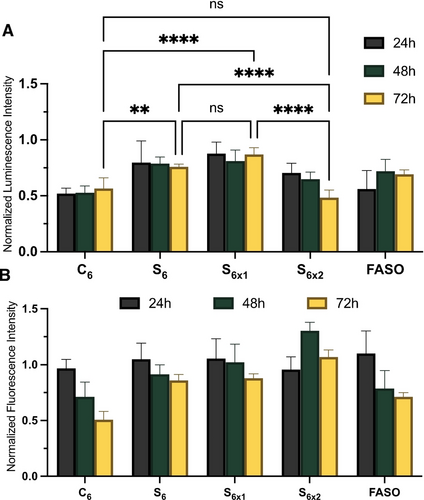
A) Firefly luciferase knockdown activity of S6, S6x, C6 and FASO after 24, 48, and 72 h of incubation in HeLa cells, without transfection agent, that are normalized to the buffer control and to the cell viability. B) Fluorescence measurement of CellTiter-Blue cytotoxicity results normalized to buffer control. Error bars represent SD of six replicates for each sample. ****: p<0.0001; ***: p<0.0002; **: p<0.0021; *: p<0.033; ns: p>0.123.
This effect is much more pronounced in S6x2, the activity of which gradually improves over time until it reaches the same level of silencing as C6 at 72 h incubation with no toxicity. This is likely the result of the higher initial cellular uptake of S6x2 as compared to S6, combined with the reduction of S6x2 and its marginally improved endosomal escape. Non-targeting and inactive controls were performed to ensure no non-specific knockdown were taking place (Figure S26A). The non-targeting control consisted of a fully phosphorothioated 2′-FANA modified gapmer with a phosphate cleavable linker, while the inactive sequence was a non-FANA, fully phosphorothioated ASO that did not contain a cleavable linker (Table S1).
Comparing C6 and S6x2, it is clear that the rate of release of the therapeutic FASO and endosomal escape of the whole construct are not bottlenecks for gene silencing. This finding is of great importance for the future design of gene silencing nanostructures: as long as the ASO therapeutic is released from its carrier, it will eventually escape the endolysosomal network and exhibit gene silencing, whether that escape is immediate or over longer periods of time. Therefore, future designs should focus on improving the overall cellular uptake of these nanostructures, and endosomal escape is not a crucial bottleneck for such constructs.
From these experiments, we showed that changing the core chemistry of self-assembled SNAs allows for fine-tuning of the overall micelle stability, small-molecule encapsulation, cellular penetration, endosomal escape, silencing ability, and toxicity. Some of these constructs reach higher silencing activities than non-modified FASO strands with lower toxicity; we anticipate that they will show improved biodistribution in vivo7, 8 compared to non-modified oligonucleotides, which are rapidly cleared.46 Crosslinked S6x2 SNA brings together an enhanced extracellular stability with intracellular dissociation, yielding structures with high unaided gene silencing activity and low toxicity.
Conclusions
In summary, we demonstrated that the core composition of self-assembled SNAs largely impacts their stability and therapeutic activity. By changing the nature of the hydrophobic core, we can modulate extracellular stability, intracellular dissociation, and self-assembly properties. Additionally, introducing disulfides into the interior of the SNA led to stimuli-responsive drug delivery vehicles that dissociate in reductive intracellular conditions. Disulfides also allowed for dynamic covalent crosslinking to resist albumin-induced dissociation.
We investigated all parameters needed for efficient gene silencing activity of self-assembled SNAs. Extracellular stability, especially with regards to SNA disassembly with serum proteins, governs cellular uptake, with stable SNAs more readily taken up than albumin-bound dissociated structures. Too high stability, however, can prevent efficient therapeutic release intracellularly (under transfection conditions). Whereas bioreducible SNAs have potent gene silencing efficacy under transfected conditions, non-reducible counterparts have significant knockdown abilities in non-transfected conditions because of their higher stability and uptake. This, however, comes at the cost of toxicity over time. Disulfide crosslinking of the SNA core brings advantageous properties both intra- and extracellularly: it enhances extracellular stability and cellular uptake, resulting in high gene silencing over time with no need for transfection agents, without compromising toxicity, and yielding an excellent candidate for unaided gene silencing.
We anticipate that these detailed investigations of the barriers faced by self-assembled structures on their way to biological activities will help toward their application in vivo, where interactions with serum proteins are especially prevalent. Moving forward, we will investigate how the different SNA constructs explored here will perform in an in vivo setting, especially with regard to their tunable response to albumin binding and consequences on biodistribution and gene silencing.
Supporting Information
Additional references were cited in the Supporting Information.47-49
Author Contributions
S. F. performed most of the biological experiments, synthesized some DNA amphiphiles, analyzed the data and co-wrote the manuscript. Q. L synthesized phosphoramidites and some DNA amphiphiles, characterized the materials, analyzed the data, and co-wrote the manuscript. A. P. designed the original system and performed initial synthesis and characterizations. J. A. performed some flow cytometry and gene silencing experiments and analyzed the data. D. S. collected and analyzed all AFM images. V. T. helped with phosphoramidite synthesis. H. F. S. designed the project, guided the interpretation of data and results discussion, and co-wrote the manuscript.
Acknowledgments
This work was funded by the Natural Science and Engineering Research Council of Canada (NSERC), Canada Foundation for Innovation (CFI) and Fonds de Recherche du Québec—Nature et Technologies (FRQNT). S. F. is thankful to NSERC for a CREATE training scholarship. Q. L. acknowledges the Swiss National Science Foundation (SNSF) for a Postdoc.Mobility fellowship (P500PN_206907). A. P. is thankful to NSERC for a PGSD fellowship. We thank Dr. Alexander Wahba for the mass spectrometry characterization.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




