Solar Hydrocarbons: Single-Step, Atmospheric-Pressure Synthesis of C2−C4 Alkanes and Alkenes from CO2
Abstract
Cobalt ferrite (CoFe2O4) spinel has been found to produce C2−C4 hydrocarbons in a single-step, ambient-pressure, photocatalytic hydrogenation of CO2 with a rate of 1.1 mmol g−1 h−1, selectivity of 29.8 % and conversion yield of 12.9 %. On stream the CoFe2O4 reconstructs to a CoFe−CoFe2O4 alloy-spinel nanocomposite which facilitates the light-assisted transformation of CO2 to CO and hydrogenation of the CO to C2−C4 hydrocarbons. Promising results obtained from a laboratory demonstrator bode well for the development of a solar hydrocarbon pilot refinery.
Introduction
The goal of carbon neutrality is motivating the transition of the global energy landscape from fossil fuels toward renewable sources integrated with CO2 recycling. Hydrogenation of CO2 using renewable energy offers sustainable routes to synthesize value-added chemicals (alkane and alkene hydrocarbons), while mitigating the greenhouse gas CO2.1 Low molecular weight alkanes and alkenes, comprising two to four carbon atoms (C2−C4), are important feedstocks for synthesizing renewable chemicals and fuels.2 Conventional synthesis of C2−C4 hydrocarbons are realized by industry-established Fischer-Tropsch synthesis (FTS), which involves the heterogeneous hydrogenation of CO at elevated pressure between 1 MPa and 3 MPa.3 Substituting CO with CO2 in FTS as a carbon source to produce hydrocarbons makes the reaction more demanding and challenging due to the difficulties related to the thermodynamic stability of CO2 and the competing formation of methane.2b, 4 CO2-modified Fischer-Tropsch synthesis (CO2−FTS) routes normally involves two consecutive reactions: the reverse water-gas shift (RWGS) reaction CO2+H2→CO+H2O (ΔH(298 K)=41 kJ mol−1), followed by the hydrogenation of CO to make hydrocarbons n CO+2 n H2→(−CH2−)n+n H2O (ΔH(298 K)=−152 kJ mol−1).4, 5 Although there are successful examples of CO2 hydrogenation to produce hydrocarbons, they generally are conducted under high pressure over a tandem system to promote conversion efficiency and overcome high kinetic barriers for C−C couplings.6 The integration of abundant and renewable solar energy into traditional thermally powered technologies has been proven as a probably promising strategy to avoid harsh reaction conditions.7 Thus, we explored the efficacy of converting CO2 into low molecular weight C2-C4 hydrocarbons under atmospheric pressure with the assistance of photo irradiation.
CoFe2O4 is also an iron-based semiconductor oxide with a narrow band gap of 1.76 eV, which could absorb the visible light efficiently and thus promote the photocatalytic reactions.12 Based on their tunable composition, structure and optical properties, cobalt ferrites are considered to be photocatalysts of choice to apply in CO2−FTS under ambient conditions assisted by solar energy.
Herein, we have discovered that CoFe2O4 can function as an efficacious catalyst precursor for enabling the CO2 hydrogenation reaction under atmospheric pressure conditions, showing notable selectivity towards C2−C4 hydrocarbons. The active sites were identified as an alloy/spinel CoFe/CoFe2O4 bimetallic heterostructure, created photothermally under in situ reaction conditions. The CoFe2O4 spinel enabled the RWGS reaction from CO2/H2 to produce CO, while the as-formed CO provided the synthon to enable the FTS over the CoFe alloy. The enhanced ability of capturing photons over the entire solar spectra for the in situ formed catalyst enabled a photothermal FTS reaction, the synergy of which contributed to a high C2−C4 rate of 1.1 mmol g−1 h−1, selectivity of 29.8 % and a CO2 conversion yield of 12.9 %.
Results and Discussion
Performance of CO2 conversion to hydrocarbons on cobalt ferrite
Initially, cobalt ferrite precursors with different Fe/Co ratios (FC1, FC2 and FC5) were synthesized by a simple hydrothermal method and then pre-treated under the in situ reaction atmosphere (H2/CO2=4) in the light at 300 °C for 10 h. The activity measurements of three samples were carried out to explore the performance of the CO2 hydrogenation to high-valued hydrocarbons with or without light. For FC2, CH4 and C2−C4 hydrocarbons could be produced above 240 °C under dark. Solar C2−C4 hydrocarbons were produced at 200 °C and significantly increased above 260 °C. An impressive C2−C4 selectivity of 29.8 % with a noteworthy production rate of 1.1 mmol g−1 h−1 and 12.9 % CO2 conversion efficiency were achieved at 300 °C (Figure 1a,b). In comparison with the other two samples, FC1 was inferior to FC2 with respect to the production of C2-C4 at the same conditions, while neither CH4 nor C2-C4 hydrocarbons were detected for FC5, which also had the lowest CO production rate (Figure S2). The relating works on CO2 hydrogenation to C2−C4 production are shown in Table S1, the selectivity of C2−C4 hydrocarbons of FC2, even conducted at atmospheric pressure in a flow mode, was comparable to those observed under higher pressures. A typical product distribution measured by GC is shown in Figure S3.
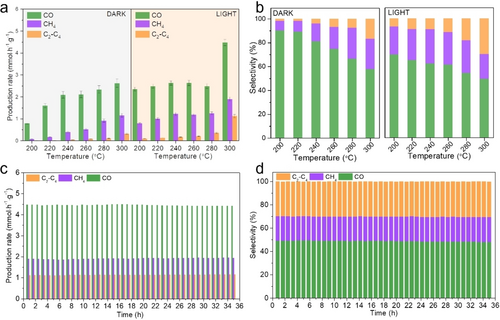
CO2 hydrogenation performance. a, b) The production rate and corresponding selectivity of CO, CH4, and C2−C4 hydrocarbons for sample FC2 with and without light irradiation in the temperature range of 200-300 °C and atmospheric pressure. c), d) Stability of FC2 over 35 hours showing the solar-powered hydrogenation of CO2 production rate and selectivity towards CO, CH4, and C2-C4, respectively (reaction conditions: H2/CO2=4, P=0.1 MPa, T=300 °C, light intensity of 40 suns).
FC2 was further used for conducting stability test. It displayed excellent stability during the long-term test under ambient pressure and illumination (Figure 1c,d). FC2 was also examined at a much harsher condition with pressure (3 MPa) in a strictly iso-thermal annular gap microreactor under industrially relevant conditions. As shown in Figure S4, CH4 and C2−C4 hydrocarbons could be produced with higher selectivity than that under lower pressure below 300 °C, and liquid C5+ species were generated when the operating temperature was raised to 350 °C. The excellent CO2 reduction ability manifested the significant potential for industrial applications.
Identification of active sites
Co and Fe were distributed uniformly in the three fresh samples (FC1, FC2 and FC5) and the atomic ratios of Fe to Co were consistent with the trend of their original feed values (Figure S5 and Table S2). The FC2 matched well with the cubic spinel structure CoFe2O4 (JCPDS #22-1086) in PXRD patterns,13 as well as FC1 (Figure S6). FC5 had both CoFe2O4 and Fe2O3, and the later was further confirmed by the Fe−O stretching mode at 485 cm−1 via ATR-FTIR spectrum (Figure S7).14 In addition, all the as-synthesized catalysts intensively absorbed light across UV/Vis-NIR region, here 250–2500 nm (Figure S8). An increased Co component resulted in a promoted optical absorption. After solar CO2 hydrogenation reactions, CoFe alloy and CoO integrated with CoFe2O4 nanospinel were detected in the spent cobalt-rich FC1 (Figure S9). Both CoFe alloy and CoFe2O4 nanospinel were measured in the spent FC2 (Figure 2a).15 And CoFe alloy could not be detected in the spent iron-rich FC5 (Figure S9). Considering the best performance of FC2, it was reasonable to infer that heterostructured CoFe alloy/CoFe2O4 nanocomposite undertook a critical role in the conversion of CO2 to hydrocarbons.
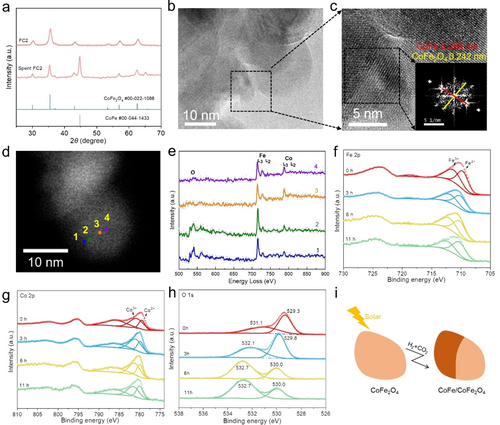
a) Powder X-ray diffraction patterns of fresh and spent FC2. b) HRTEM image of spent FC2. c) HRTEM image derived from (b) (inset image is FFT pattern). d), e) High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image of spent FC2 and corresponding O K-edge, Fe L-edge and Co L-edge EELS spectra at different positions. f), g), h) Time-dependent in situ XPS results. Fe 2p (f), Co 2p (g) and O 1s (h) peaks of spent FC2 under 0.1 MPa, 300 °C, CO2+H2 (2.5 sccm, and H2/CO2=4, light intensity of 40 suns). i) The illustration of cobalt ferrite structural transformation.
To identify the structure and composition, the TEM and HRTEM images of the spent FC2 are shown in Figure S10 and Figure 2b,c. The lattice spacings of 0.286 nm and 0.242 nm, observed on a single spent FC2 particle, corresponded to the (100) of CoFe nanoalloy and the (222) of CoFe2O4.16 The HRTEM results indicate the CoFe closely adhered to the CoFe2O4, forming the composite heterostructure. The particle size of fresh and spent FC2 showed a little shrinkage due to its in situ reduction and the formation of CoFe alloy, as shown in Figure S10.
STEM-EELS analysis shows the EEL O K-edge, Fe L-edge and Co L-edge spectra in Figure 2d,e, respectively. The L2 (L3) features in the metal spectra arise from 2p1/2 (2p3/2)→3d transitions, while the O K-edge comes from the transitions from the O 1s→2p states, which are hybridized with Fe 3d (Co 3d) orbitals due to covalent Fe−O (Co−O) bonding.17 The O K pre-peak signal was not detected at some points (from Point 1 to Point 4), further confirming the partial reduction of the cobalt ferrite and the formation of a metallic alloy phase.18 Both of Fe L3/L2 and Co L3/L2 white-line intensities were recorded across the sample (Table S3), indicating the uniform distribution of metal Fe and Co in the particle. So the metallic component was CoFe alloy instead of the single phase of Co or Fe. The STEM-EELS line scan results of a CoFe nanoalloy pre-fabricated by H2 reducing are shown in Figure S11. There was no O pre-peak observed in the spectra, which in turn confirmed the in situ reduction of CoFe2O4 during the reaction. The average Fe L3/L2 and Co L3/L2 white line intensity ratios of the pre-fabricated CoFe alloy and spent FC2 are summarized in Table S4. Moreover, the element mapping results showed that Fe and Co elements were distributed uniformly in the pre-fabricated CoFe alloy (Figure S12). The atomic ratio of Co : Fe in the pre-fabricated CoFe alloy was 1 : 1.72 obtained from EDX analysis, in accordance to the ICP result of the fresh FC2 (Table S2), so we can speculate that the ratio of Co and Fe in CoFe alloy is around 1 : 1.7.
To simulate the reaction condition, chemical states of the catalyst in atmospheric H2 or CO2+H2 were analyzed by in situ XPS under light irradiation. Obviously, the catalyst surface within the detection limit, around several nanometers, remained a metal oxide layer according to the Fe 2p and Co 2p spectra, however, they measured in both atmospheres showed an increased proportion of bivalent component over the time on stream (Figure 2f–h, Figure S13 and Tables S5 and S6).19 Meanwhile, in the O 1s spectra, the signal diagnostic of the lattice oxygen exhibited a decreased intensity versus time due to the removal of lattice oxygen atoms in the reductive H2+CO2 and H2 atmosphere. Both of above analysis indicated the gradual in situ reduction process of FC2 on stream. The illustration of cobalt ferrite structural transformation is shown in Figure 2i.
In situ XAS was conducted both in H2 and H2+CO2 atmospheres to further explore the structural evolution of the cobalt ferrite. Heating samples at 300 °C was employed to match the reaction temperature, and at 350 °C to simulate photothermal heating. In Fe and Co XANES spectra (Figure 3a,b), reduction of cobalt ferrite was indicated qualitatively by a gradual intensity increase in the pre-edge feature (ca. 7113 and 7712 eV, respectively) and an intensity decrease of the white-line feature (ca. 7130 and 7725 eV, respectively). Fe showed some initial reduction (albeit slow) at 300 °C under H2, virtually no change was observed in the Co spectra, suggesting that the Fe sites were more active than Co sites to be reduced. As the temperature was increased to 350 °C, both Co and Fe in the oxide were transformed from cationic states to metallic form (Fe0 and Co0) to produce CoFe alloy. After CO2 was purged into the reaction, reduction of Fe or Co was to some extent inhibited, consistent with the in situ XPS result.
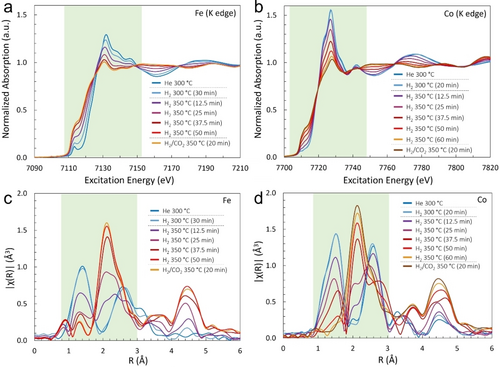
In situ temperature-dependent XAS spectra of the XANES (a, b) and EXAFS (c, d) at the Fe K-edge and Co K-edge of FC2. “x min” indicates the number of minutes the sample has spent at the indicated temperature. Data were collected in the flow rate of 2.5 sccm totally and H2/CO2 ratio is 4.
In Fe and Co Fourier transform EXAFS spectra (Figure 3c,d), the peak located at ca. 1.4 Å corresponded to the nearest-neighbor metal-oxygen (M−O) bonding, i.e., Fe−O or Co−O. And the peak located at ca. 2.6 Å corresponded primarily to the metal-metal scattering interactions M(Oh)−O−M(Oh), i.e., Fe(Oh)−O−M(Oh) or Co(Oh)−O−M(Oh), where M could be Fe or Co. Another peak around 3.2 Å represented the interaction of Fe(Td)−O−M(Td) or Co(Td)−O−M(Td).20 By comparing the relative strength of M(Oh)−O−M(Oh) to M(Td)−O−M(Td), it could conclude that Fe atoms occupied both Oh and Td positions, and most Co atoms were located at the Oh centers, indicative of an inverse spinel structure for initial FC2.20 Very little change was observed in either peak of the M−O and the M(Oh)−O−M(Oh) following reduction at 300 °C. While obvious reductions of M(Td)−O−M(Td) were observed in both Co and Fe spectra, therefore, the cation reductions mainly occurred at the Oh centers at first. Accordingly, more Fe cations in Td sites than Co cations and the more sensitive capacity of Fe to construct interactions with H2, thus, possibly resulted in the CO production at low-temperature regions. On increasing the temperature to 350 °C, M−O and M−(Oh)−O−M(Oh) peaks began to decrease in intensity, while the M−M interactions in CoFe alloy, located at ca. 2.2 Å, were rapidly increased in intensity. Consistent results were obtained through the coordination numbers (CNs) for representative scattering (Figures S14–S16 and Tables S7 and S8).
Based on the structural and electronic characterizations, how CoFe2O4 was reconstructed after the activation procedure was figured out. Fe3+ and Co2+ in the lattice of CoFe2O4 were collapsed and aggregated to form the CoFe alloy phase in the reductive atmosphere, resulting in the active CoFe/CoFe2O4 heterostructure. Meanwhile, the surface of the catalyst intensively interacted with the CO2 that could prevent the complete reduction and remained oxidative state.
Synergistic effect of CoFe alloy and CoFe2O4 in CO2 hydrogenation
The synergistic effects of the two components in the in situ formed CoFe/CoFe2O4 heterostructure should play essential roles in solar C2−C4 hydrocarbons production.21 Pure CoFe nanoalloy fabricated from CoFe2O4, as proven by PXRD results (Figure S17), was first investigated as a comparison. Even though CoFe alloy had better light absorption property than cobalt ferrite (Figure S18), little amounts of CO (0.085 mmol g−1 h−1) and CH4 (0.002 mmol g−1 h−1) and no C2−C4 hydrocarbons were produced under light condition (Figure S19), far from the performance of CoFe/CoFe2O4. It was reasonable to infer pure CoFe alloy might be difficult to initiate the RWGS reaction for CO formation, thus the successive hydrogenation was inevitably limited. These results were consistent with the inferior C2−C4 hydrocarbons production of FC1 compared to that of FC2, because of more proportion of CoFe alloy in the spent FC1.
Obvious pieces of evidence of synergistic effect between CoFe2O4 and CoFe alloy were obtained from the CO2 adsorption behaviors. When exposure CO2 on the pristine CoFe2O4 at room temperature (Figure 4a), infrared fingerprint modes at 1220 and 1413, and 1620 cm−1 assigned to the adsorbed CO2 species were observed in DRIFTS.22 The intermediates gradually transformed to formates when the temperature increased to 200 °C with emerging peaks at 1520 and 1320 cm−1, indicating the easy activation of CO2 over CoFe2O4. In sharp contrast, no signals belonging to adsorbed species were detected over pure CoFe alloy (Figure 4b). Even introducing the reactive gases (H2/CO2=4), the signals of adsorbed CO2, formate intermediates or hydrocarbon products were not observed from room temperature to 300 °C (Figure 4b). The CO2-TPD results further confirmed above conclusions (Figure 4c). Two desorption peaks were observed for FC2 and CoFe/CoFe2O4, while no signals appeared for CoFe alloy. CO2 adsorption and activation were the critical steps before subsequent hydrogenation procedures.23 Therefore, the relatively poor CO2 adsorption capacity resulted in the inferior CO2 hydrogenation activity over CoFe alloy. And these results hinted that CoFe2O4 was an essential component for the first RWGS step, and CoFe alloy should participate in the subsequent CO hydrogenation processes.24 Their synergies drove the reaction to produce C2−C4 under the ambient condition with light irradiation.
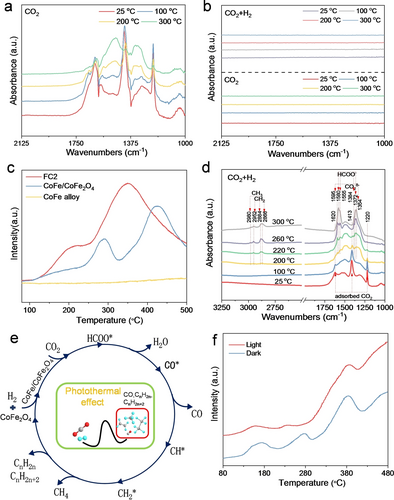
a) In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) study of CoFe2O4 nanoparticles under a flow of gaseous CO2 at different temperatures with light irradiation in the temperature range 25–300 °C. b) DRIFTS study of CoFe alloy under a flow of gaseous H2 and CO2 (H2/CO2=4) or only CO2 at different temperatures with light irradiation in the temperature range of 25-300 °C. c) CO2-TPD patterns of FC2, CoFe/CoFe2O4 and CoFe nanoalloy. d) DRIFTS studies of FC2 under a flow of gaseous H2 and CO2 (H2/CO2=4) at different temperatures with light irradiation in the temperature range 25-300 °C. e) Possible reaction pathway of FC2 for CO2 hydrogenation. f) Hydrogen programmed temperature reduction (H2-TPR) results of FC2 in light and dark conditions.
Reaction pathway
The in situ DRIFTS were applied to provide a molecular-level understanding of CO2 activation over CoFe/CoFe2O4. In the presence of both H2 and CO2, the peak intensity of adsorbed CO2 decreased with the rising temperature and disappeared around 200 °C (Figure 4d). New vibrational modes at 1354 and 1595 cm−1 associated with the formation of carboxylate species emerged,22c, 25 implying an effective conversion of adsorbed CO2 species in the hydrogenation process. In addition, several modes at 1372, 1384, 1555 and 1580 cm−1 appeared beyond 220 °C, which attributed to the newly formed formate species.26 Carboxylate and formates gradually transformed to CO and continued the FTS process, leading to longer chain hydrocarbons.27 Concurrently, C−H stretching vibrations of methyl and methylene groups in the region of 2500–3000 cm−1 belonging to various hydrocarbon species appeared beyond 200 °C and grew with the temperature, consistent with the results of temperature-dependent C2−C4 production activities.28
Accordingly, the collected results of the catalytic performance and DRIFTS studies allowed us to draw the possible reaction pathways over the CoFe/CoFe2O4 nanocomposite involved in the hydrogenation of CO2 to CO, CH4 and C2−C4, as shown in Figure 4e. Under the reductive atmosphere, partial CoFe2O4 was initially reduced by H2 to form the CoFe/CoFe2O4 heterostructured nanocomposite. Then CO2 adsorbed on the surface of CoFe2O4, and reacted with H2 to make formate, which further hydrogenated to generate CO and H2O (the RWGS pathway). Subsequently, the CoFe alloy in the interface of CoFe/CoFe2O4 heterostructure nanocomposite will contribute to C−C coupling in the chain growth of hydrocarbons.29
The role of solar energy
The advantage of light-assisted CO2 hydrogenation on FC2 has been illustrated by the above results. To investigate the role of solar energy, hydrogen programmed temperature reduction (H2-TPR) measurements were conducted and three peaks in the 100–400 °C were recorded in both light and dark conditions (Figure 4f). Thereinto, peaks around 170 and 280 °C in the dark shifted towards lower temperatures at 160 and 230 °C respectively in the light, indicating solar light enabled the reduction of CoFe2O4 at a lower external temperature. This might be ascribed to the solar light-induced photothermal effect, causing the improvement of local temperature. In addition, the performance of FC2 at higher temperature (400 °C) with the 17.9 % selectivity of C2−C4 and 13.0 % CO2 conversion, was slightly lower than that at 300 °C under solar light (Figure S20), further revealing the importance of the solar-induced thermal heating effect.8g, 30
The temperature at the surface of the catalyst during the reaction was monitored using an infrared (IR) camera (Figure S21), which illustrated a approximate 17–19 °C increase under light illumination. To further explore the contribution of the photothermal effect to the reaction process, a 2D reactor model was built in an in-house MATLAB®-based multi-physics simulation environment. Continuum methods were applied to map light irradiation and heat transport properties, and therewith estimated the bulk temperature distribution in the catalyst bed. Sensitivity analysis showed that the maximum bulk temperature rise over the reactor body can be expected at about 20 °C depending mostly on the optical and thermal properties of the catalyst bed (Figure S22). Our simulation results represented a qualitative indication of the heating effect of the incident light itself over the catalyst bed, although the estimated extent of the bulk temperature rise cannot fully explain the observed differences between light and dark. In our case, the photo-induced local heating might fluctuate significantly and result in an inhomogeneous local temperature distribution depending on the microstructure of the catalyst bed, but our simulation indeed indicated the significance of such photothermal heating, a topic that deserves more attention in future studies. Consequently, the solar energy not only facilitated the in situ formation of CoFe/CoFe2O4, but also benefited the CO2 hydrogenation reaction through the photothermal effect.
Conclusion
In this study, a cobalt ferrite spinel (CoFe2O4) is demonstrated to function as a photocatalyst for the hydrogenation of CO2 to low molecular hydrocarbons (C2−C4) at atmospheric pressure, with a rate of 1.1 mmol g−1 h−1 and selectivity of 29.8 % towards C2−C4 hydrocarbons with a CO2 conversion yield of 12.9 %. The combined results from PXRD, SEM-TEM, XPS, XAS, EELS and DRIFTS indicate that the active catalyst under reaction conditions is comprised of nano alloy CoFe and nano spinel CoFe2O4 components, working synergistically to enable solar FTS. In situ XAS demonstrates that Fe3+ and Co2+ in the lattice of CoFe2O4 undergoes reductive nucleation and growth to form the CoFe alloy phase that is integrated with the remaining unreduced CoFe2O4 phase, resulting in the active CoFe/CoFe2O4 heterostructure. H2-TPR measurements in the dark and the light, combined with MCRT/FEM simulations, reveal that a photothermal effect plays an important role in facilitating the partial reduction of the CoFe2O4 to the active nanocomposite CoFe/CoFe2O4 catalyst as well as the subsequent production of hydrocarbons. Results from a scaled laboratory demonstrator bodes well for the future development of a pilot solar hydrocarbon refinery.
Acknowledgments
D.J. wishes to acknowledge the financial supports of National Key R&D Program of China (No. 2018YFB1502000). G.A.O. acknowledges the financial support of the Ontario Ministry of Research Innovation (MRI); Ministry of Economic Development, Employment and Infrastructure (MEDI); Ministry of the Environment and Climate Change (MOECC); Low Carbon Innovation Fund (LCIF), Ontario Centre of Excellence Solutions 2030 Challenge Fund; Alexander von Humboldt Foundation; Imperial Oil, University of Toronto Connaught Innovation Fund; Connaught Global Challenge Fund and the Natural Sciences and Engineering Research Council of Canada (NSERC). W. Sun acknowledges Zhejiang University Global Partnership Fund. R. Song acknowledges the support from the China Scholarship Council (201706280298). Thanks go to Mireille Ghoussoub, Paul Kant, Hannah Kirsch, Uta Gerhards, Micheal Klumpp and Roland Dittmeyer for helping in the TGA tests, experiments design, H2-TPR measurements and the 2D MCRT/FEM simulations.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Research data are not shared.




