Photo-RAFT Polymerization for Hydrogel Synthesis through Barriers and Development of Light-Regulated Healable Hydrogels under NIR Irradiation
Abstract
We report an aqueous and near-infrared (NIR) light mediated photoinduced reversible addition–fragmentation chain transfer (photo-RAFT) polymerization system catalyzed by tetrasulfonated zinc phthalocyanine (ZnPcS4−) in the presence of peroxides. Taking advantage of its fast polymerization rates and high oxygen tolerance, this system is successfully applied for the preparation of hydrogels. Exploiting the enhanced penetration of NIR light, photoinduced gelation is effectively performed through non-transparent biological barriers. Notably, the RAFT agents embedded in these hydrogel networks can be reactivated on-demand, enabling the hydrogel healing under NIR light irradiation. In contrast to the minimal healing capability (<15 %) of hydrogels prepared by free radical polymerization (FRP), RAFT-mediated networks display more than 80 % recovery of tensile strength. Although healable polymer networks under UV and blue lights have already been established, this work is the first photochemistry system using NIR light, facilitating photoinduced healing of hydrogels through thick non-transparent barriers.
Introduction
Photoinduced reversible-deactivation radical polymerization (photo-RDRP) systems1 confer excellent spatiotemporal control over the synthesis of well-defined polymers under light irradiation. Among them, photoinduced reversible addition-fragmentation chain-transfer (photo-RAFT) systems utilizing photocatalysts (PCs)2 and photoinitiators3 have received significant interest due to their ability to employ under a broad range of wavelengths and at times without the need for deoxygenation.2a, 2h, 3a, 4 Although UV and visible light mediated RAFT systems are well-established,2a-2d, 3a, 3b NIR light mediated systems are relatively rare,1o, 2f, 2h especially in water.2g, 5 This can be attributed to the scarcity of PCs with NIR absorption and water solubility. Although some aqueous NIR light mediated RAFT polymerizations were recently introduced, prior deoxygenation5b and slow polymerization rates2g limited their broader applications.
Aqueous media in polymerization is preferred over organic solvents for environmental and biological benefits.5a, 6 Despite their underutilization, NIR light mediated chemistry offers some exceptional advantages, including the ability to induce chemical reactions through barriers and at increased penetration depths compared to UV and visible light, which provides an avenue for potential applications in manufacturing and biomedical fields.1k, 1o, 2h, 6b, 7 For example, photopolymerizations were successfully applied in injectable hydrogel systems, minimizing operation wounds and conferring spatiotemporal control of gelation through the skin.6a, 8 In recent years, taking advantage of enhanced penetration of NIR light, efficient photoinduced gelation through barriers has been demonstrated in NIR photopolymerization systems.8a, 9 In 2016, the Lee group utilized gold nanorods as the sensitizer to induce photothermal polymerization of poly(ethylene glycol) diacrylate (PEGDA) in the presence of initiators under NIR laser irradiation, resulting in successful transdermal gelation and good viability of the transplanted cells.8a In 2020, Chen and co-authors developed a NIR photopolymerization system for hydrogel fabrication, enabling non-invasive 3D bioprinting in vivo.9 However, no NIR photochemistry systems have demonstrated the reactivation of polymer networks for hydrogel healing. Photoinduced healing of hydrogel networks through barriers is an exciting concept, which may have a broad range of applications in the medical field and material engineering. Exploiting the NIR light mediated photo-RAFT systems demonstrated in this work, reactivation of RAFT units, i.e., thiocarbonylthio groups, embedded in hydrogel networks is possible, which enables hydrogel healing through non-transparent barriers under NIR light. Although UV and blue light mediated healable polymer networks have been established,2b, 3c, 10 to the best of our knowledge the current work presents the first NIR-induced photochemistry system. Furthermore, compared with previous NIR light mediated hydrogel systems where upconverting nanoparticles (UCNPs) and high-energy laser devices were required,9, 11 in our work, a commercial PC and low-energy light-emitting diodes (LEDs) were used, opening the possibility to use this process without the requirement of specialized equipment and strict safety protocols.
In this study, tetrasulfonated zinc phthalocyanine (ZnPcS4−) is utilized as a PC to mediate photo-RAFT polymerization in the presence of peroxides under NIR LEDs. The oxygen tolerance of this system is enhanced further by adding triethanolamine (TEOA) as a reducing agent, allowing the preparation of hydrogels at ambient temperature without deoxygenation. High penetration of NIR wavelengths promotes RAFT-mediated gelation through thick barriers. In addition, tensile testing demonstrates that the incorporation of RAFT agents in hydrogel networks enhanced the elongation at break in comparison with hydrogels prepared by conventional free radical polymerizations (FRP). Under NIR light, the healing performance of RAFT-mediated hydrogels was investigated by reactivation of thiocarbonylthio groups embedded in the networks. In contrast to RAFT-mediated networks with high recovery of mechanical properties, FRP-mediated hydrogels only result in minimal strength recovery under NIR light, indicating the crucial role of the RAFT agent. Top- and cross-sectional views of the healed interface of hydrogels obtained by SEM exhibit a complete recovery of the samples, confirming the efficiency of this NIR healing process. Moreover, the dependence of mechanical property recovery on irradiation time is reported. Excitingly, this photo-RAFT system facilitates the reactivation of thiocarbonylthio groups in polymer networks under NIR wavelengths, which is demonstrated as an efficient platform for photoinduced healing of hydrogels through barriers.
Results and Discussion
Aqueous photo-RAFT polymerization under NIR light
Under NIR light irradiation, tetrasulfonated zinc phthalocyanine (ZnPcS4−), a water-soluble PC (Scheme 1B and Supporting Information, Figure S1), was employed to photosensitize hydrogen peroxide (H2O2) in the presence of trithiocarbonate (TTC) RAFT agent, for the photoinitiation of RAFT polymerization in aqueous solution (Scheme 1A). N,N-dimethylacrylamide (DMAm) and 4-((((2-carboxyethyl)thio)carbonothioyl)thio)-4-cyanopentanoic acid (CTCPA) (Scheme 1C) were selected as model monomer and TTC owing to their good solubility in water. Firstly, a series of control experiments were performed with and without deoxygenation, demonstrating the need for NIR light, ZnPcS4−, and H2O2. Low monomer conversions were observed in the absence of either these species or light irradiation (Supporting Information, Table S1, #2, 3, 5, 7, 8, and 10). In comparison with the photo-RAFT polymerization after deoxygenation, the reaction without deoxygenation displayed a slower polymerization rate (a lower apparent propagation rate coefficient, kpapp) (Supporting Information, Figure S2A, kpappnon-degas=0.70 h−1 versus kpappdegas=0.92 h−1). This result was attributed to the consumption of radicals by the presence of oxygen. However, photo-RAFT with and without deoxygenation exhibited excellent control towards the preparation of poly(N,N-dimethylacrylamide) (PDMAm) polymers (Supporting Information, Table S1, #1 and 6; Figure S2B) as indicated by low dispersity (Đ<1.15) and good agreement between theoretical (Mn, theo) and experimental (Mn, GPC) molecular weights. Moreover, in the absence of the RAFT agent, oxygen inhibition was also observed in the non-degassed reaction, resulting in lower conversions at analogous time points (Supporting Information, Table S1, #4 and 9).
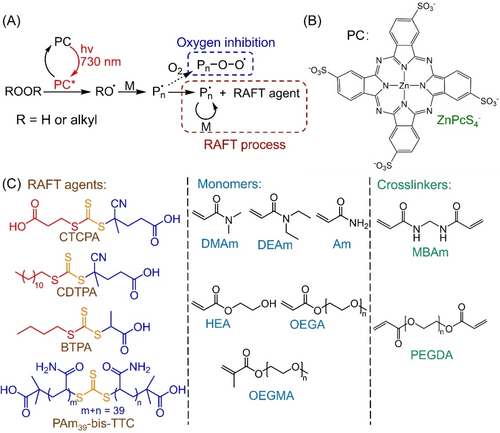
(A) Proposed mechanism of photo-RAFT polymerization utilizing (B) tetrasulfonated zinc phthalocyanine (ZnPcS4−) to photosensitize peroxides (Supporting Information, Scheme S1) under NIR light. (C) Trithiocarbonate (TTC) RAFT agents, monomers, and crosslinkers used in this work: 4-((((2-carboxyethyl)thio)carbonothioyl)thio)-4-cyanopentanoic acid (CTCPA); 4-cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl] pentanoic acid (CDTPA); 2-(butylthiocarbonothioylthio)propanoic acid (BTPA); polyacrylamide bis-trithiocarbonate (PAm39-bis-TTC); N,N-dimethylacrylamide (DMAm); N,N-diethylacrylamide (DEAm); acrylamide (Am); 2-hydroxyethyl acrylate (HEA); oligo(ethylene glycol) methyl ether acrylate (OEGA, Mn=480 g mol−1); oligo(ethylene glycol) methyl ether methacrylate (OEGMA, Mn=300 g mol−1); N,N′-methylenebis(acrylamide) (MBAm); poly(ethylene glycol) diacrylate (PEGDA, Mn=700 g mol−1).
Besides H2O2, other peroxides, including tert-butyl hydroperoxide (TBHP), urea hydrogen peroxide (UHP), and di-tert-butyl peroxide (DTBP), were tested (Supporting Information, Scheme S1). Well-defined polymers (Đ<1.2; Supporting Information, Figure S3) were successfully synthesized by photo-RAFT polymerization in the presence of all three peroxides (Supporting Information, Table S2) under NIR light irradiation. In comparison with the polymerization in the presence of UHP and DTBP, TBHP-mediated photo-RAFT polymerization was much faster (Supporting Information, Table S2, #3–5). Overall, H2O2 still displayed the fastest rate among these peroxides (Supporting Information, Table S2, #2). Therefore, H2O2 was selected in this photo-RAFT system for the following experiments.
To demonstrate the versatility of this system, two other TTC RAFT agents, including 4-cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid (CDTPA) and 2-(butylthiocarbonothioylthio)propanoic acid (BTPA) (Scheme 1C) were investigated in systems without deoxygenation and using a relatively high monomer concentration (50 v %) in water to enable the solubilization of RAFT agents. BTPA-mediated photopolymerization demonstrated better control (Supporting Information, Table S3 and Figure S4) than CDTPA-mediated system as evidenced by a lower dispersity (Đ BTPA≈1.1 versus Đ CDTPA≈1.2). This may be attributed to the more hydrophobic Z-group of CDTPA, i.e., n-dodecyl group, than the n-butyl group of BTPA. However, CTCPA-mediated photopolymerization displayed a much higher monomer conversion than BTPA after 2 h NIR irradiation (Supporting Information, Table S3, #1 and 3). As a result, CTCPA was selected as a RAFT agent in the following experiments.
To investigate the role of H2O2 in this photo-RAFT system, we conducted a series of experiments using different ratios of H2O2 relative to CTCPA from 0.05 to 0.5 (Supporting Information, Figure S5 and Table S4). Using 0.05 equivalents of H2O2, a long induction period was observed (Supporting Information, Figure S5, orange line), with no monomer conversion within 1 h and sluggish polymerization thereafter. The conversion reached a plateau at ≈29 % monomer conversion after 3 h. Interestingly, a shorter inhibition period (≈0.5 h) and a faster polymerization rate were observed when using an H2O2 ratio of 0.1 (Supporting Information, Figure S5, purple line). These results indicated that a higher H2O2 concentration promoted photo-RAFT polymerization with a faster rate and a shorter inhibition period. However, the kinetic plot deviated from pseudo-first order behavior, indicating a low radical concentration in these conditions. At 0.5 and 1.0 equivalents of H2O2, a linear relationship between ln([M]0/[M]t) and irradiation time was observed, indicating a constant concentration of radicals (Supporting Information, Figure S5).
The consumption of H2O2 was also monitored in situ by 1H NMR spectroscopy using DMF as an internal standard during the photo-RAFT polymerization (Figure 1A; Supporting Information, Figure S6). Interestingly, H2O2 consumption only occurred under NIR light irradiation (Figure 1A). As observed via 1H NMR spectroscopy, the integral of the peak corresponding to H2O2 decreased from 0.96 to 0.72 after 1 h of NIR light irradiation, then stayed essentially constant (integral=0.71) throughout a 2 h dark period. Upon reexposure to NIR light for 1 h, the H2O2 consumption resumed as indicated by a further decrease in the H2O2 peak integral from 0.71 to 0.39 (Figure 1A and Supporting Information, Figure S6). This result is consistent with previous publications examining zinc phthalocyanines for photodynamic therapy: the ability of zinc phthalocyanines to photosensitize peroxides and generate hydroxyl radicals was demonstrated in these works.12 To further confirm the formation of hydroxyl radicals, terephthalic acid (TA), which is commonly used as the probe to detect hydroxyl radicals (Supporting Information, Figure S7A),13 was added into reaction mixtures containing ZnPcS4− and H2O2 in water. After NIR light irradiation, the fluorescence spectrum of this mixture displayed a significantly increased peak at ≈440 nm (Supporting Information, Figure S7B, green curve), which corresponds to the fluorescent product of the reaction between TA and hydroxyl radicals. Meanwhile, no change in fluorescence was observed in the control experiment in the dark (Supporting Information, Figure S7, orange curve), indicating that hydroxyl radicals were only generated under light irradiation in the presence of H2O2 and ZnPcS4− (Scheme 1A).
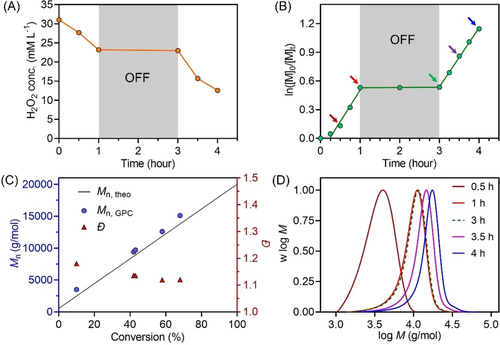
(A) Consumption of H2O2 in photo-RAFT polymerization under NIR light exposure. Note: 64.8 mM DMF was added into the reaction solution as an internal standard to quantify H2O2 concentration. (B) Demonstration of temporal control of photo-RAFT polymerization in the presence of ZnPcS4− and H2O2 under the irradiation of NIR light (λmax=730 nm; I=61.6 mW cm−2) with reaction stoichiometry of [DMAm] : [CTCPA] : [H2O2] : [ZnPcS4−]=200 : 1 : 1 : 0.01 at [DMAm]=50 v % in water without deoxygenation. Note: the arrows correspond to molecular weight distributions (MWDs) in (D). (C) Evolution of Mn and Đ versus monomer conversion from the same reaction in (A). (D) Evolution of the normalized MWDs versus reaction time from the same reaction in (A).
Temporal control over the aqueous photo-RAFT polymerization process was also demonstrated, as evidenced by the absence of monomer conversion when the light was turned off (Figure 1B). As the reaction was placed again under NIR light, photopolymerization resumed with little change in kpapp (kpapp before=0.62 h−1 versus kpapp after=0.65 h−1). This temporal control can be explained by the absence of formation of initiating species in the dark, as H2O2 can only be photosensitized by the excited state of ZnPcS4− under light (Scheme 1A). Furthermore, we observed a good agreement between Mn, theo and Mn, GPC and a decreasing Đ from ≈1.2 to ≈1.1 with increasing monomer conversion (Figure 1C). Additionally, with increasing light irradiation time, molecular weight distributions (MWDs) of the synthesized PDMAm shifted toward higher molecular weights, indicating the controlled character of this photo-RAFT system (Figure 1D). Moreover, the chain extension of a PDMAm macroRAFT agent was performed by adding fresh DMAm to the reaction mixture and exposing the new mixture to NIR light. A clear shift in the MWD for PDMAm-b-PDMAm compared to the initial PDMAm was observed by GPC (Supporting Information, Figure S8), indicating the high fidelity of thiocarbonylthio groups during the photopolymerization. The high end group fidelity was also supported by 1H NMR spectroscopy with retention of the characteristic signal at 5.1 ppm attributed to the CH adjacent to the thiocarbonylthio group. The ratio of the CH signal to the CH3 protons from the n-dodecyl Z-group is close to the expected value (Supporting Information, Figure S9).
Subsequently, we tested different targeted degrees of polymerization (DPs) in this oxygen-tolerant photo-RAFT system by fixing the concentrations of monomer, ZnPcS4− and H2O2 and changing RAFT agent concentrations. Well-defined PDMAm was successfully synthesized with narrow MWDs (Đ<1.25) at target DPs of 200, 600, and 2000 (Supporting Information, Table S5 and Figure S10). However, the synthesized polymers displayed a broad MWD (Đ=1.51) when the target DP was 5000. This can be attributed to the low concentration (0.87 mM) of RAFT agents used in this experiment. To investigate the versatility of this photo-RAFT system toward various monomers, the family and type of water-soluble monomers were expanded. Under NIR light irradiation without requiring prior deoxygenation, N,N-diethylacrylamide (DEAm), 2-hydroxyethyl acrylate (HEA), oligo(ethylene glycol)methyl ether acrylate (OEGA, Mn=480 g mol−1), and oligo(ethylene glycol) methyl ether methacrylate (OEGMA, Mn=300 g mol−1) were successfully polymerized with narrow MWDs (Đ<1.25) in the presence of ZnPcS4−, H2O2 and CTCPA in water (Supporting Information, Table S6 and Figure S11).
Hydrogel synthesis regulated by NIR light
After establishing aqueous photo-RAFT polymerization, we decided to exploit these systems for the fabrication of hydrogels owing to their good performance in the presence of oxygen. A polyacrylamide TTC macroRAFT agent (Scheme 1C, PAm39-bis-TTC) was synthesized (Supporting Information, Figure S12) and used as macroRAFT in photo-RAFT polymerization of hydrogels, owing to its high water solubility. As PAm39-bis-TTC did not dissolve in most organic solvents, a chain extension experiment (Supporting Information, Table S4, #4) was performed on PAm39-bis-TTC using DMAm as a monomer to enhance solubility in dimethylacetamide (DMAc) for GPC characterization. A well-controlled block copolymer PAm-b-PDMAm was observed with symmetric MWDs (Supporting Information, Figure S4) and low Đ (1.11), indicating the living character of the original PAm39-bis-TTC macroRAFT agent. Hydrogels were prepared by chain extension of PAm39-bis-TTC using oligo(ethylene glycol) methyl ether acrylate (OEGA) as the monomer and poly(ethylene glycol) diacrylate (PEGDA) as the crosslinker in the presence of H2O2, and ZnPcS4− without deoxygenation in water under NIR light irradiation (Scheme 1C). Although successful gelation was observed in 40 mins (Figure 2A, yellow line), the top layer of the reaction remained liquid. Under light irradiation for 100 mins, the monomer conversion of the bottom gel layer (≈800 μL) reached over 80 % as indicated by FTNIR spectroscopy, while the monomer conversion of the top liquid layer (≈200 μL) stayed below 10 % (Figure 2A, top right; Supporting Information, Figure S13A). This incomplete gelation can be attributed to the diffusion of oxygen from the vial headspace to the top layer of the reaction, leading to the partial inhibition of the photopolymerization. For the photopolymerization of DMAm in the previous section, a 0.8 mL flat cuvette with a cross-section of 1 cm×2 mm was employed, whereas a 5 mL cylindrical glass vial with a diameter of 1.0 cm was used for the synthesis of hydrogels. Although photo-RAFT polymerization of DMAm displayed a good oxygen tolerance in flat cuvettes, the rapid diffusion of oxygen from the headspace resulted in more significant inhibition and retardation of the polymerization (Supporting Information, Figure S14) in the top layer using the glass vial for hydrogel synthesis. To confirm this assumption, the same reaction after deoxygenation was performed. As expected, a faster polymerization was achieved with 90 % monomer conversion obtained after 60 mins, resulting in the formation of complete hydrogel without the presence of a liquid layer (Supporting Information, Figure S13B). However, deoxygenation requires stringent protocols such as the use of a glovebox, which limits the applicability of these approaches. We also tried to synthesize hydrogel without deoxygenation in a silicone mold rather than in a glass vial. After exposure to NIR light for 10 h, partial gelation was still observed (Supporting Information, Figure S15A), indicating that the oxygen diffusion hindered the gelation.
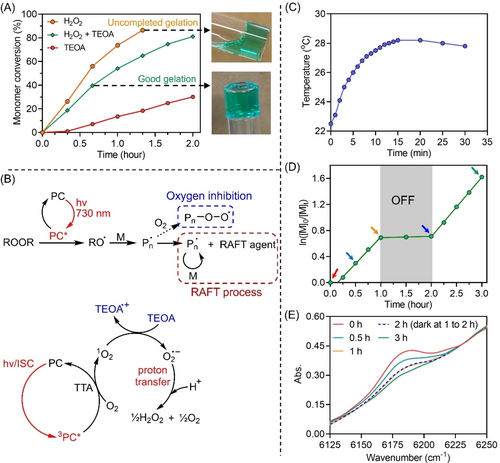
(A) Kinetics of ZnPcS4− mediated photo-RAFT polymerization of hydrogels under NIR light (λmax=730 nm; I=61.6 mW cm−2) without deoxygenation in various experimental conditions. Fixed reaction stoichiometry of [OEGA] : [PEGDA] : [PAm39-bis-TTC] : [H2O2] : [TEOA] : [ZnPcS4−]=200 : 50 : 1 : (0 or 20) : (0 or 20) : 0.01 was used at [OEGA]=0.4 M in water. (B) Proposed mechanism of ZnPcS4− mediated photo-RAFT polymerization of hydrogel in the presence of TEOA. (C) The temperature change of photopolymerization under NIR light using the same experimental condition as (A) in the presence of H2O2 and TEOA. (D) Demonstration of temporal control of ZnPcsS4− mediated photo-RAFT polymerization of hydrogels without deoxygenation under NIR light using the same reaction condition as (A) in the presence of H2O2 and TEOA. (E) Decreasing peak of acrylate double bond monitored by FTNIR with increasing light exposure time from the same reaction in (D).
Under light irradiation and in the presence of excited ZnPcS4− (3PC*), oxygen (O2) can be transformed into singlet oxygen (1O2) by triplet-triplet annihilation (TTA; Figure 2B).14 Previous investigations have demonstrated that the addition of triethanolamine (TEOA) can react with 1O2 producing superoxide (O2⋅−) and H2O2 species.4a, 15 As a result, dissolved oxygen can be rapidly eliminated via this mechanism. Inspired by these early contributions, TEOA was added to the hydrogel solution to improve oxygen tolerance. Excitingly, after placing the reaction mixture under NIR light irradiation for 40 mins, complete gelation was observed in the presence of TEOA and ZnPcS4− (Figure 2A, green line). A similar result was observed for the hydrogel synthesis in a silicone mold where no liquid remained (Supporting Information, Figure S15B). To investigate the role of TEOA in this photo-RAFT system, various control experiments were conducted (Supporting Information, Table S7). In the absence of ZnPcS4− or NIR light irradiation (Supporting Information, Table S7, #2 and 7), no monomer conversion was observed in the presence of TEOA, which is consistent with previous control experiments (Supporting Information, Table S1, #7 and 8). This means ZnPcS4− and light are required for the activation of this photo-RAFT polymerization rather than TEOA. Interestingly, in the absence of H2O2, photopolymerization in the presence of ZnPcS4− and TEOA reached 30 % monomer conversion in 2 h under NIR light irradiation (Figure 2A, red line; Supporting Information, Table S7, #3). Compared with the fast polymerization with H2O2 (kpapp=0.68 h−1; Supporting Information, Figure S16A), photo-RAFT polymerization in the presence of TEOA without deoxygenation displayed a slower apparent polymerization rate (kpapp=0.38 h−1). As H2O2 can be photosensitized by ZnPcS4− to generate initiating species (Figure 2B), the slower polymerization rate in the TEOA system can be attributed to the generation of a small amount of H2O2 by the reaction between TEOA and 1O2 (Figure 2B, bottom).4a, 15 This was confirmed by a slow formation of H2O2 determined by 1H NMR spectroscopy (Supporting Information, Figure S16).
In addition to PAm39-bis-TTC, PDMAm macroRAFT agent (Mn=15 100; Đ=1.13) was also tested (Supporting Information, Figure S17A) for the synthesis of hydrogel in the presence of ZnPcS4−, H2O2, and TEOA under NIR irradiation. Successful gelations were observed after 1 h light irradiation when using the PDMAm macroRAFT agent (Supporting Information, Figure S17B).
Previous NIR polymerization systems employed for the fabrication of hydrogels used lasers with high light intensity, which led to noticeable photothermal effects with an increase in the reaction temperature that can exceed 40 °C.8a, 11b On the contrary, our system employs LEDs with low light intensity, promoting NIR photopolymerization under mild conditions (Figure 2C). Experimentally, the reaction temperature went up from ≈22 to ≈26 °C under irradiation (λmax=730 nm; I=61.6 mW cm−2) for 5 mins, followed by a slow increase to ≈28 °C in the next 10 mins. Then, the reaction temperature dropped slightly and stayed at ≈28 °C between 15 and 30 mins. In addition, temporal control over photoinduced gelation was demonstrated by turning OFF the light (Figure 2D), displaying negligible monomer conversion during the dark period (Figure 2E). Photopolymerization resumed when the NIR light was turned ON again with no significant change in polymerization rate (kpapp before=0.83 h−1 versus kpapp after=0.89 h−1).
Photoinduced gelation through non-transparent barriers
Notably, in comparison with visible light (λ=400–700 nm), NIR wavelengths possess enhanced light penetration (Figure 3A),1k, 1o, 2g, 9 which can be exploited to mediate polymerization through non-transparent barriers for the fabrication of hydrogels with potential biomedical applications. To emphasize the enhanced penetration of NIR wavelengths compared to visible light, the light intensity of green (λmax=530 nm) and NIR light (λmax=730 nm) was measured before and after passing through barriers with different thicknesses (Figure 3B). Through 2.5 and 5.0 mm pig skin, the light intensity (I) of green light decreased to 2.3 % and 0.3 % of the initial value (I0). By contrast, in the case of 730 nm NIR light, 21.3 % and 10.6 % of the initial light intensity were able to pass through 2.5 and 5.0 mm pig skin, respectively.
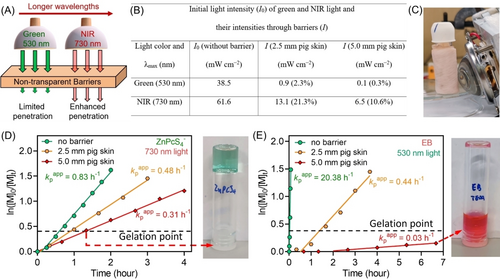
(A) Longer wavelengths possessing enhanced penetration through non-transparent barriers. (B) Reduction in light intensity of green (λmax=530 nm) and NIR (λmax=730 nm) light after passing through 2.5 and 5.0 mm pig skin. (C) Experimental setup of NIR light mediated hydrogel synthesis through 2.5 mm pig skin. (D) ln([M]0/[M]t) versus irradiations time plots of ZnPcS4− mediated photo-RAFT polymerization in the presence of H2O2 and TEOA under NIR light irradiation (λmax=730 nm; I=61.6 mW cm−2) after passing through 2.5 and 5.0 mm pig skin with reaction stoichiometry of [OEGA] : [PEGDA] : [PAm39-bis-TTC] : [H2O2] : [TEOA] : [ZnPcS4−]=200 : 50 : 1 : 20 : 20 : 0.04 at [OEGA]=0.4 M in water. (E) ln([M]0/[M]t) versus irradiations time plots of Erythrosin B (EB) mediated photo-RAFT polymerization in the presence of TEOA under green light irradiation (λmax=530 nm; I=38.5 mW cm−2) after passing through 2.5 and 5.0 mm pig skin with reaction stoichiometry of [OEGA] : [PEGDA] : [PAm39-bis-TTC] : [TEOA] : [EB]=200 : 50 : 1 : 1 : 0.04 at [OEGA]=0.4 M in water.
To demonstrate the applicability of this system to produce hydrogels through thick barriers, photo-RAFT polymerization was performed through biological barriers with various thicknesses (Figure 3C). In the presence of 2.5 mm pig skin, kpapp of Erythrosin B (EB) mediated photo-RAFT polymerization (Supporting Information, Scheme S2) under green light decreased from 20.38 to 0.44 h−1 (Figure 3E), which corresponds to 2 % of the original polymerization rate. By contrast, ZnPcS4−-mediated NIR photo-RAFT system through 2.5 mm pig skin (Figure 3D) displayed 58 % of the kpapp without barrier (kpapp (with barrier)=0.83 vs kpapp (without barrier) 0.48 h−1). Therefore, NIR photopolymerization system exhibited a much smaller decrease in kpapp than the green light system when pig skin was placed between the light source and the reaction. Upon increasing the barrier thickness to 5.0 mm (pig skin), kpapp of the EB system under green light was determined to be 0.03 h−1 which corresponds to only 0.015 % of the original kpapp, while the NIR system exhibited a kpapp equal to 0.31 h−1, corresponding to 37 % of the value without barrier. For the NIR photopolymerization through 5.0 mm thick pig skin, hydrogels were successfully prepared despite an increase in gelation time from ≈40 to ≈80 mins (Figure 3C, red lines). By contrast, the green light mediated hydrogel system (Supporting Information, Scheme S2) using EB and TEOA performed poorly in the presence of thick barriers. Although there is rapid gelation (Figure 3E, green line; kpapp=20.38 h−1; 80 % monomer conversion in 5 mins) without barriers under green light exposure which is expected as our previous results,16 the polymerization under green light passing through 5.0 mm pig skin performed at a sluggish rate (Figure 3E, red line; kpapp=0.03 h−1) and no gelation was observed under irradiation after 7 h (Figure 3E, right).
Effect of RAFT polymerization on hydrogel properties
Previous investigations indicate that polymer networks prepared by RAFT polymerizations possess more homogeneous microstructures than networks made by FRP.17 Highly crosslinked polymeric nanogels with compact structures are generated at the early stages of FRP (Figure 4A, top first), due to the rapid consumption of pendant double bonds via intramolecular polymerization in the presence of propagating radicals. As demonstrated in previous work by atomic force microscopy (AFM), scanning electron microscope (SEM), and light scattering,18 these nanogels first remain isolated at the early stage of the polymerization. In a later stage of the polymerization, they can react via intermolecular crosslinking, eventually forming a heterogeneous network (Figure 4A, top second). In contrast to FRP, RAFT polymerization allows an equilibrium between propagating species and dormant polymer chains to be established, which enables more even growth of all polymer chains throughout the polymerization (Figure 4A, bottom top). The efficient chain transfer in the RAFT system allows diffusion of polymer chains and intermolecular crosslinking, producing more homogeneous polymer networks (Figure 4A, bottom second).19
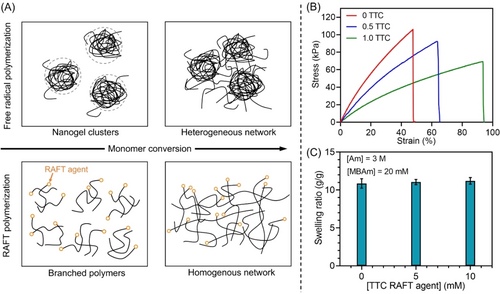
(A) Formation of heterogeneous hydrogel network via free radical polymerization and formation of homogeneous hydrogel network via RAFT polymerization. (B) Stress-strain curves of hydrogels synthesized using reaction stoichiometry of [Am] : [MBAm] : [PAm39-bis-TTC] : [H2O2] : [TEOA] : [ZnPcS4−]=(280.5 and 261) : 2 : (0.5 and 1) : 8 : 8 : 0.015 for photo-RAFT polymerization at [Am unit]=5 M under NIR light (λmax=730 nm; I=25.6 mW cm−2) and [Am] : [MBAm] : [TEOA] : [EB]=300 : 2 : 8 : 0.015 for FRP at [Am unit]=5 M under green light (λmax=730 nm; I=13.5 mW cm−2). (C) Swelling ratios in 1× PBS (pH=7.4) of hydrogels containing different concentrations of PAm39-bis-TTC after 24 h using the same reaction stoichiometry of (B) at [Am]=3 M.
To investigate the effect of the RAFT agent on the mechanical properties of hydrogels, samples containing various concentrations of RAFT agent were synthesized and analyzed via tensile testing. A hydrogel system consisting of acrylamide (Am) and N,N′-methylenebis(acrylamide) (MBAm) was selected, owing to its higher strength than the previously used polyacrylate networks. ZnPcS4− mediated photo-RAFT polymerizations were performed under NIR light for 10 h, enabling nearly full vinyl bond conversions (>99 % by FTNIR spectroscopy). Meanwhile, due to the sluggish FRP observed in ZnPcS4− system (Supporting Information, Table S1, #9; Table S7, #6), FRP-mediated hydrogels with comparable vinyl bond conversion (>99 %) were synthesized using EB and TEOA under green light irradiation. The tensile strength of hydrogel samples increased with decreasing concentration in thiocarbonylthio groups (1.0 ratio of TTC RAFT agent relative to 300 Am units: 68.0±4.2 kPa, 0.5 ratio of TTC: 89.2±9.4 kPa; Figure 4B; Supporting Information, Table S8), and the sample without addition of RAFT agent possessed the highest tensile strength at 106.0±6.0 kPa. In contrast, the elongation at break increased for hydrogels with higher RAFT agent concentrations (1.0 TTC RAFT agent: 92.0±7.7 %; 0.5 TTC: 61.4±3.4 % versus 0 TTC: 45.0±2.3 %; Figure 4B; Supporting Information, Table S8). Overall, lower tensile strength and higher elongation at break were observed in RAFT-mediated hydrogel networks compared to FRP-mediated networks, which was in agreement with the property of RAFT-mediated 3D printed materials in previous contributions.3c Owing to the efficient chain transfer and diffusion in RAFT polymerization, more homogeneous polymer networks were prepared (Figure 4A, bottom second), benefiting the polymer chains uncoiling.17a In addition, previous contributions reported that the addition of RAFT agents into hydrogel networks significantly reduces shrinkage stress and enhances relaxation of the polymer networks.20
The swelling properties of hydrogels containing various concentrations of the RAFT agent were determined. Hydrogels prepared by NIR photo-RAFT were swollen in 1× PBS (pH=7.4) for 24 h to obtain the equilibrium swollen weight (Supporting Information, Figure S18), and the dry weights of samples were obtained after freeze-drying. Overall, in comparison with FRP-mediated networks, polymer networks containing thiocarbonylthio groups showed no evident change in swelling ratios (Figure 4C), which was consistent with the previous reports by Takai group17b and Konkolewicz group.21 In addition, the effect of crosslinker concentrations on swelling ratios of RAFT-mediated hydrogel networks was investigated. As expected, swelling ratios of hydrogel networks decreased with increasing concentrations of crosslinkers (Supporting Information, Figure S19).
NIR light mediated hydrogel healing
A unique advantage of RAFT polymerization is the facile reactivation of RAFT agents embedded in polymer networks. In the presence of fresh monomers, the polymer chains can be extended, resulting in the modification of materials synthesized by RAFT polymerizations.2b, 3c, 10a, 22 The living property conferred by the RAFT group has also been exploited to self-heal damaged polymer networks. Although self-healing hydrogel systems have been established widely,23 many of them suffer from complex experimental procedures or low recovery of mechanical strength.24 In this study, hydrogel networks were healed efficiently under NIR light irradiation by reactivation of thiocarbonylthio groups in the presence of PC, monomers, and crosslinkers, achieving high tensile strength recovery. Although previous contributions demonstrated the self-healing of polymer network under UV light by reactivation of RAFT agents,3c, 10a, 10b these high-energy photons lead to unwanted side reactions, such as the degradation of thiocarbonylthio groups.25 Besides UV light, Johnson's group developed a hydrogel system that was healed under blue light in the presence of PC.2b However, compared with NIR wavelengths, blue light displays low penetration through barriers, limiting its applicability to non-transparent systems.
We performed hydrogel healing experiments by reactivating RAFT agents under NIR light (Figure 5A). Taking advantage of enhanced light penetration of these longer wavelengths, the photoinduced-healing process of hydrogels is accessible through thick barriers (Figure 3A), which may present potential applications in broad fields. In addition, as previously demonstrated, difunctional RAFT agents consisting of symmetric leaving R groups can display more efficient healing capability owing to the location of the thiocarbonylthio group within the polymer chains.3c, 10a, 22a Therefore, PAm39-bis-TTC (Scheme 1C), a difunctional macroRAFT agent with great water solubility was used for hydrogel fabrication. Experimentally, hydrogel samples containing a 1 ratio of TTC RAFT agent relative to 300 Am units were synthesized by ZnPcS4−-mediated photo-RAFT polymerization under NIR light with nearly complete conversion of vinyl bonds (>99 %). Subsequently, the sample was cut into two pieces. In our first attempts, the healing process was performed by placing the two parts in contact in the hydrogel mold consisting of a silicone frame and two glass plates under NIR light irradiation at room temperature and in an open-air environment. Unfortunately, we only observed moderate healing under NIR light for 3 h, as indicated by 17±3 % recovery of tensile strength and 9±1 % recovery of elongation at break (Supporting Information, Table S8, #3). This can be attributed to the low amount of residual monomers and crosslinkers in the hydrogel sample limiting the healing process, although some thiocarbonylthio units can be reactivated under NIR light. This result was in agreement with Johnson and co-workers’ work, which reported poor healing capability of a RAFT-mediated hydrogel under blue light in the presence of the PC only.2b Consequently, a small amount (≈20 μL) of hydrogel precursor solution consisting of [Am]: [MBAm]: [H2O2]: [TEOA]: [ZnPcS4−]=300 : 2 : 8 : 8 : 0.015 at [Am]=5 M without RAFT agent was introduced at the cut interface prior to healing under NIR light for 3 h (Figure 5C). Excitingly, tensile testing of the healed hydrogel samples (Figure 5F; Supporting Information, supplementary video) revealed 84±4 % recovery of tensile strength and 76±2 % recovery of elongation at break (Figure 5D, green curve; Figure 5E; Supporting Information, Table S8, #2). The proposed mechanism of healing process is presented in Scheme S3. In our process, we added a solution containing ZnPcS4− and H2O2 to the interfaces of cut hydrogels. Under NIR irradiation, the initiating hydroxyl radicals can be generated, which can propagate in the presence of residual monomers (Supporting Information, Scheme S3A) to generate propagating radicals (Pn⋅ and Pm⋅). These radicals can enter into the RAFT process (Supporting Information, Scheme S3A–B), leading to the formation of intermediate radical species. Upon fragmentation of these intermediate radical species, the polymer networks containing thiocarbonylthio groups are reactivated to yield radicals (Supporting Information, Scheme S3B–C). These radicals can propagate (Supporting Information, Scheme S3C–D) or transfer to thiocarbonylthio groups. The healing process is induced by the crosslinking between polymer chains (Supporting Information, Scheme S3D–E) and the RAFT chain transfer process (Supporting Information, Scheme S3E–F) between active chains and dormant chains, resulting in the formation of new networks. Moreover, a lower healing efficiency with 41±7 % recovery of tensile strength and 29±5 % recovery of elongation at break (Figure 5D, blue curve; Figure 5E; Supporting Information, Table S8, #5) was observed in the samples prepared using a lower ratio of RAFT agents (the 0.5 ratio of thiocarbonylthio units). This decreased healing capability can be attributed to a lower concentration of thiocarbonylthio groups embedded in polymer networks, resulting in less reactivation of polymer chains at hydrogel interfaces, which is consistent with previous results.3c, 10c Tensile tests of healed samples demonstrated that high concentrations of RAFT agents in hydrogel networks were essential to improve tensile strength recovery (Figure 5D, blue and green curves). In contrast to samples containing thiocarbonylthio units, FRP-mediated hydrogels (Figure 5B) displayed minimal healing ability in the presence of the same hydrogel precursor solution, displaying only less than 16±4 % and 13±2 % recovery of stress and elongation at break (Figure 5D, red curve; Figure 5E; Supporting Information, Table S8, #7). This poor healing capability demonstrates that the polymer chains produced in FRP-mediated hydrogels cannot be reactivated under NIR irradiation and the RAFT end group is necessary for the healing process (Supporting Information, Scheme S3).
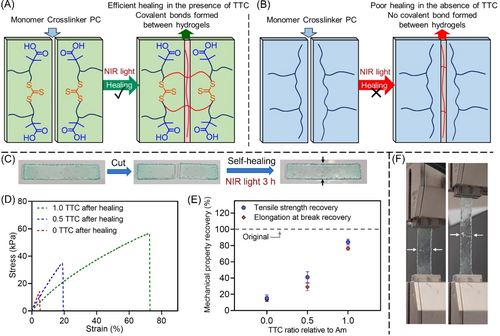
(A) The healing process of hydrogels containing RAFT agents by photo-RAFT polymerization under NIR light. (B) Limited healing of FRP-mediated hydrogels under NIR light. (C) Digital photos of the hydrogel healing experiment. (D) Stress-strain curves of healed hydrogels containing various concentrations of PAm39-bis-TTC under NIR light for 3 h (Stress-strain curves of original hydrogels before healing shown in Figure 4B.) Note: Original hydrogels were synthesized using reaction stoichiometry of [Am] : [MBAm] : [PAm39-bis-TTC] : [H2O2] : [TEOA] : [ZnPcS4−]=(280.5 and 261) : 2 : (0.5 and 1):8 : 8 : 0.015 for photo-RAFT polymerization at [Am unit]=5 M under NIR light (λmax=730 nm; I=25.6 mW cm−2) and [Am] : [MBAm] : [TEOA] : [EB]=300 : 2 : 8 : 0.015 for FRP at [Am unit]=5 M under green light (λmax=730 nm; I=13.5 mW cm−2). Then cut samples were healed under NIR light (λmax=730 nm; I=113.0 mW cm−2) for 3 h. (E) Tensile property recovery of healed hydrogels containing various concentrations of TTC RAFT agent (0, 0.5, and 1 ratios relative to 300 units of Am) under NIR light for 3 h. (F) Experimental setup of tensile testing of the healed hydrogel. Note: the healed sample in the image and supplementary video was synthesized using the 1.0 TTC ratio in Figure 5D, and the healing time is 3 h.
After drying, healed hydrogel samples were characterized by scanning electron microscopy (SEM; Figure 6B–D). The top view of sample interfaces indicated successful healing (Figure 6B). To further demonstrate the success of the healing process, cross-sectional views of healed interfaces were imaged in SEM (Figure 6C–D) by cutting the samples in the direction perpendicular to the healing line (Figure 6A). The successful binding of the two separated hydrogel pieces was evidenced by the absence of cracks in the cross-sectional view of the healed material. Moreover, after swelling in PBS for 24 h, the healed hydrogels effectively swelled and showed no signs of rupture at the healed interface or elsewhere. (Supporting Information, Figure S20).
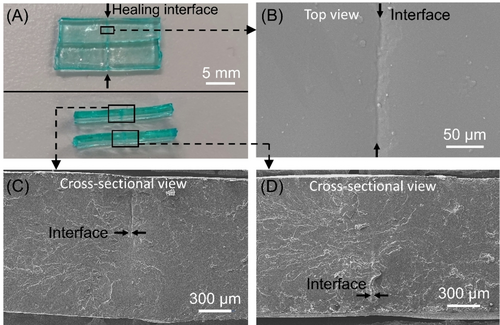
(A) Digital photos and SEM images of the (B) top and (C–D) cross-sectional views of the healing interfaces (healing time=3 h) of the dried hydrogel. Note: Original hydrogels were prepared using reaction stoichiometry of [Am] : [MBAm] : [PAm39-bis-TTC] : [H2O2] : [TEOA] : [ZnPcS4−]=261 : 2 : 1 : 8 : 8 : 0.015 at [Am unit]=5 M in water under NIR light (λmax=730 nm; I=25.6 mW cm−2). Note: cut hydrogels were healed in the silicone mold by adding 20 μL precursor water solution consisting of [Am] : [MBAm] : [H2O2] : [TEOA] : [ZnPcS4−]=300 : 2 : 8 : 8 : 0.015 ([Am]=5 M) to the contact area under NIR light irradiation (λmax=730 nm; I=113.0 mW cm−2) for 3 h.
The effect of NIR light irradiation time on the recovery of tensile properties was also investigated (Figure 7A–B and Table S9). As expected, tensile strength and elongation at break of the healed samples exhibited higher recovery with increasing NIR light irradiation time. Under NIR light irradiation for 1 h, 44±4 % of strength and 30±2 % elongation were recovered (Figure 7A, red curve). These were increased further to 68±3 % and 59±3 %, respectively, after 2 h NIR irradiation (Figure 7A, yellow curve). Pleasingly, near quantitative recovery of strength (99±8 %) and elongation at break (93±5 %) were observed after 5 h irradiation (Supporting Information, Figure S7A, blue curve).
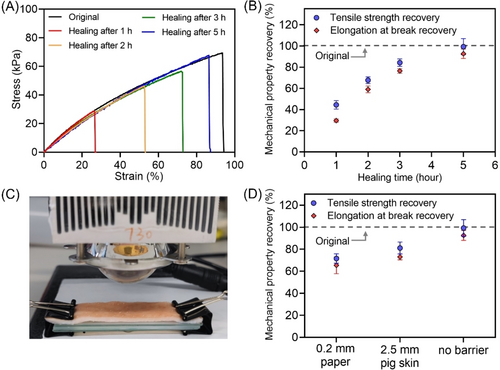
(A) Stress-strain curves of original and healed hydrogels at various healing time points (1, 2, 3, and 5 h) under NIR light irradiation. Note: Original hydrogels were prepared using reaction stoichiometry of [Am] : [MBAm] : [PAm39-bis-TTC] : [H2O2] : [TEOA] : [ZnPcS4−]=261 : 2 : 1 : 8 : 8 : 0.015 at [Am unit]=5 M in water under NIR light (λmax=730 nm; I=25.6 mW cm−2). Cut hydrogels were healed in the silicone mold by adding 20 μL precursor water solution under NIR light (λmax=730 nm; I=113.0 mW cm−2. (B) Tensile property recovery of healed hydrogels at various healing time points (1, 2, 3, and 5 h) under NIR light. (C) Experimental setup of hydrogel healing in a mold consisting of a silicone frame and two glass plates under NIR light passing through 2.5 mm pig skin. (D) Mechanical property recovery of hydrogel samples after the photoinduced healing through various non-transparent barriers (0.2 mm paper and 2.5 mm pig skin) under NIR irradiation for 5 h. Note: hydrogel samples were synthesized under NIR irradiation for 5 h using the same conditions in (A).
In the previous sections, the preparation of hydrogels through barriers (Figure 3) and NIR-mediated hydrogel healing (Figure 5) were demonstrated. Owing to the enhanced penetration of NIR light and the reactivation capability of thiocarbonylthio groups, we decided to investigate the photoinduced healing of hydrogels through non-transparent barriers using paper and pig skin as the synthetic and biological barriers (Figure 7C). In the presence of barriers, the photoinduced healing method was the same as the previous method and was performed by adding 20 μL reaction solution to the gel interfaces in a mold consisting of a silicone frame and two glass plates, then irradiating with NIR light. Excitingly, 81±5 % recovery of tensile strength and 73±3 % recovery of elongation at break were observed for healing performed through a 2.5 mm pig skin barrier (Figure 7D; Supporting Information, Table S10) under NIR irradiation for 5 h. When 0.2 mm print paper was placed between the sample and light source after 5 h, tensile strength and elongation at break of healed samples were 72±4 % and 65±8 % of the original values, respectively (Figure 7D; Supporting Information, Table S10). The photoinduced healing process allowed high recovery of hydrogel tensile strength through various non-transparent barriers, providing an avenue for potential applications in manufacturing and biomedical fields.
Conclusion
In summary, an aqueous photo-RAFT polymerization regulated by NIR light was developed using ZnPcS4− as the PC in the presence of peroxide compounds. Various polymers, including polyacrylamide, polyacrylate, and polymethacrylate, were successfully synthesized in a controlled manner without prior deoxygenation. Owing to its high oxygen tolerance and the enhanced penetration of NIR light, this photo-RAFT system was applied for the preparation of hydrogels, enabling efficient gelation through thick barriers. Furthermore, tensile testing demonstrated that RAFT-mediated hydrogel networks exhibited enhanced elongation at break in comparison with FRP-mediated hydrogels, which can be attributed to more homogeneous networks. Exploiting this NIR photo-RAFT system, the thiocarbonylthio groups embedded in hydrogel polymer chains can be reactivated by NIR light for healing. Consequently, RAFT-mediated networks exhibited high recovery (>90 %) of mechanical properties under NIR light, while FRP-mediated hydrogels only displayed minimal recovery. This efficient healing process can be attributed to covalent bonds formed across two pieces of RAFT-mediated hydrogel samples. The absence of cracks after healing was confirmed by SEM and swelling experiment. Taking advantage of the enhanced light penetration of NIR wavelengths and the high retention of RAFT groups, photoinduced healing of hydrogels through barriers was demonstrated.
Acknowledgments
C. B. acknowledges the Australian Research Council (ARC) for his Australian Laureate Fellowship (ARC FL220100016). Open Access publishing facilitated by University of New South Wales, as part of the Wiley - University of New South Wales agreement via the Council of Australian University Librarians.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




