Morphological Profiling Identifies the Motor Protein Eg5 as Cellular Target of Spirooxindoles
Abstract
Oxindoles and iso-oxindoles are natural product-derived scaffolds that provide inspiration for the design and synthesis of novel biologically relevant compound classes. Notably, the spirocyclic connection of oxindoles with iso-oxindoles has not been explored by nature but promises to provide structurally related compounds endowed with novel bioactivity. Therefore, methods for their efficient synthesis and the conclusive discovery of their cellular targets are highly desirable. We describe a selective RhIII-catalyzed scaffold-divergent synthesis of spirooxindole–isooxindoles and spirooxindole–oxindoles from differently protected diazooxindoles and N-pivaloyloxy aryl amides which includes a functional group-controlled Lossen rearrangement as key step. Unbiased morphological profiling of a corresponding compound collection in the Cell Painting assay efficiently identified the mitotic kinesin Eg5 as the cellular target of the spirooxindoles, defining a unique Eg5 inhibitor chemotype.
The closely related oxindole- and isooxindole fragments frequently occur in biologically active natural products (NPs) (Figure 1a).1 For instance, Corallocin C contains isooxindole and indole fragments linked via monopodal connectivity,2 Indirubin is a bis-oxindole alkaloid,3 and Staurosporine carries a bis-indole which is fused to an isooxindole.4 However, nature has not explored the spirocyclic combination of these NP-fragments. Since the combination of common NP-fragments in alternative arrangements and with different connectivity may yield novel bioactive chemical matter with unexpected bioactivity,5 we investigated a new scaffold-divergent synthesis of spirocyclic oxindoles and explored their biological activity.
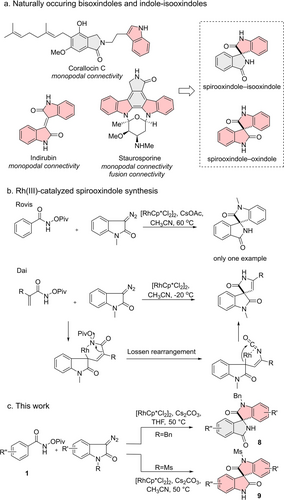
Previous and this work on RhIII-catalyzed spirooxindole synthesis.
For spirooxindole synthesis,6 for instance 1,3-dipolar cycloadditions,7 oxidative rearrangements8 and de novo synthesis have emerged as prominent methods.9 Rovis et al. reported that spirooxindoles, in principle, can be synthesized by a [RhCp*Cl2]2 catalyzed formal [4+1] cyclization.10 However, the substrate scope remained unexplored (Figure 1b). Dai et al. discovered the involvement of the Lossen rearrangement11 in the sequence when N-pivaloyloxy acrylamides were used (Figure 1b),11d however, a mechanistic explanation for the observed selectivity was not provided.
In the analysis of novel small molecule bioactivity,12 unbiased morphological profiling approaches, like the Cell Painting assay (CPA), may be particularly advantageous.13 In general, such methods employ the similarity of compound fingerprints to guide the determination of mode of action (MoA).14 However, the identification of precise molecular targets by means of the CPA has rarely been possible.15
Here we describe the discovery of a scaffold-divergent method for the selective synthesis of spirooxindole–isooxindoles and spirooxindole–oxindoles. Morphological profiling of the compound collection in the CPA and subsequent bioactivity analysis enabled the direct identification of the mitotic kinesin Eg5 as the molecular target of the spirooxindoles as a unique Eg5 inhibitor chemotype.
Screening of conditions enabled selective synthesis of spirooxindole–isooxindole 8 a9c and spirooxindole–oxindole 9 a from N-pivaloyloxy benzamide 1 a and different diazooxindoles 5 a and 7 a, respectively (Table S1). N-benzyl diazooxindole 5 a as the substrate yielded the annulation product 8 a (91 % yield, isomer ratio 8 a:S7>20 : 1) in THF, whereas in acetonitrile in the presence of the electron-withdrawing methanesulfonamide (7 a), a Lossen-rearrangement led to highly selective formation of 9 a in high yield (82 % yield, isomer ratio S10:9 a<1 : 20, see Scheme 1, for a detailed discussion see Table S1).
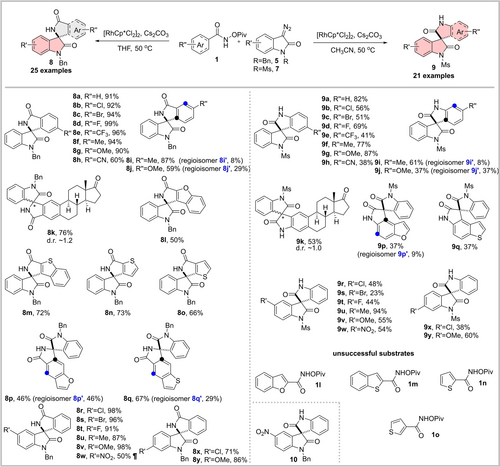
Substrate scope for scaffold-divergent synthesis of spirooxindoles. ¶ Lossen rearrangement product 10 was also isolated in 41 % yield from the reaction. d.r. diastereomeric ratio.
Subsequent exploration of substrate scope (Scheme 1) unraveled that both divergent synthesis routes tolerate diverse substituents on the N-pivaloyloxy aryl amides 1 and diazooxindoles. Notably, when 5-nitro diazooxindole 5 g was used as the substrate, the Lossen rearrangement product 10 was isolated in 41 % yield along with 50 % yield of the direct annulation product 8 w (Figure S1). In addition, furan or thiophene-based aryl amides substituted at C2 or C3 position (1 l–1 o) did not induce the formation of the Lossen rearrangement product when reacting with diazooxindole 7 a, which might be attributed to the incompatibility of the fused 5-membered ring with the stereoelectronic requirements of the rearrangement and the geometry of the intermediate. When the amide substituent was placed at C5 position of benzofuran or benzothiophene, the Lossen rearrangement proceeded to afford the products 9 p and 9 q in moderate yields.
The divergent synthesis outcome may be rationalized by the mechanism shown in Figure 2 (for mechanistic study see Figure S2). Initially, C−H activation catalyzed by [RhCp*Cl2]2 yields the rhodacycle 11, which reacts with the diazooxindole via carbene migratory insertion to yield intermediate 12. When benzylated diazooxindole 5 a is used, the reductive elimination is preferred and yields annulation product 8 a. However, the intermediate formed from N-methanesulfonyl diazooxindole 7 a does not undergo the reductive elimination and a Lossen rearrangement can occur. The resulting isocyanate can react with the C−Rh species intramolecularly to yield rearranged scaffold 9 a.

Proposal for a plausible reaction mechanism.
Biologically active compounds can be identified using target or phenotype-based assays that detect the modulation of a predefined target or a biological process.12 In contrast, profiling approaches allow the simultaneous capturing of hundreds of cellular features, which are not related to one particular target or phenotype of interest. This enables the monitoring of bioactivity and various targets in an unbiased manner. The biological activity of the spirooxindoles was investigated by means of unbiased morphological profiling in the Cell Painting assay (CPA).13 Cell Painting may enable the determination of modes of action and mechanisms of action, however, direct target identification by means of the CPA has rarely been achieved. We recently introduced a new CPA “subprofile analysis” method which greatly improves the prediction of mode and mechanism of action and rests on the definition of thus far ten bioactivity clusters describing the modulation of AKT/PI3K/MTOR, Aurora kinases, BET, DNA synthesis, HDAC, HSP90 and tubulin or processes like lysosomotropism/cholesterol homeostasis regulation (LCH), protein synthesis and uncoupling of the mitochondrial proton gradient.14 Importantly, the MoA space that is detectable using CPA has constantly been expanded to enable a broad coverage of various targets using one single assay.15a, 16
To profile the compound collection, U2OS cells were treated with the compounds at different concentrations for 20 h and then stained with six dyes to detect the nucleus, mitochondria, endoplasmic reticulum, Golgi/plasma membrane, F-actin, and RNA.17 Image analysis resulted in fingerprints consisting of 579 features that characterize the influence of the compounds on the cellular phenotype. An induction value, describing the fraction of altered features in %, serves as a measure of bioactivity and compounds were considered active if induction was higher than 5 %. The similarity of fingerprints as a measure of biosimilarity, was calculated based on Pearson's correlation distance. Fingerprints are considered similar if their biosimilarity is higher than 75 %.
Whereas most of the spirooxindoles displayed an induction higher than 5 % at 30 μM and/or 50 μM, only compounds 8 s, 8 u, 8 v and 8 k showed induction >40 % at 10 μM (Figure 3a and b and Table S2). Annulated spirooxindole-isooxindoles 8 displayed significantly higher induction than spirooxindole-oxindoles 9. Compound 8 k can be seen as a structural combination of estrone and low-induction spirooxindole 8 a (Table S2). The high induction for 8 k is dominantly caused by the estrone scaffold, as 8 k and 9 k share high biosimilarity despite different spirooxindole scaffolds (Figure 3c).
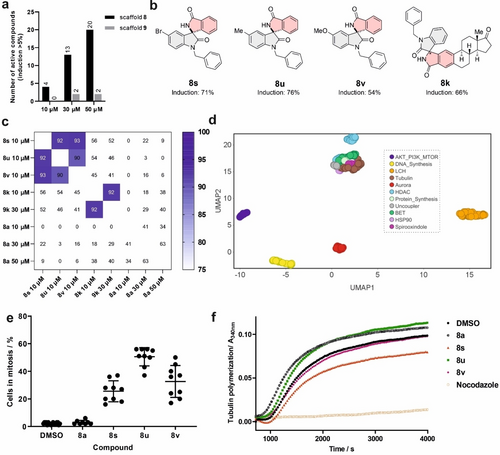
Cell Painting analysis of spirooxindoles. a) Activity in the CPA was determined using the induction values for compounds at different concentrations. b) Structures of the most active compounds 8 s, 8 u, 8 v and 8 k and the respective induction at 10 μM. c) Cross-biosimilarity between 8 s, 8 u, 8 v, 8 k, 9 k and 8 a at various concentrations. d) Location of 8 s, 8 u and 8 v in the bioactivity cluster plot using UMAP, which is a data visualization tool to reduce dimensions. Non-normalized data with 15 neighbors. e) Spirooxindoles 8 s, 8 u and 8 v induce mitotic arrest in U2OS cells. Cells were treated with the compounds for 24 h prior to staining and detection of phospho-histone H3 as a marker of mitosis and with 4′,6-diamidino-2-phenylindole (DAPI) to visualize DNA. Data are mean values ± SD of three biological replicates. Compound concentration: 10 μM. f) Influence on in vitro tubulin polymerization. Nocodazole (2 μM) was used as a control for a microtubule-destabilizing agent. Compound concentration: 20 μM. Data are representative of three biological replicates.
Spirooxindoles 8 s, 8 u and 8 v displayed high cross-biosimilarity (Figure 3c) and may share a similar MoA. They were not biosimilar to the low-induction compound 8 a (Figure 3c), which can serve as a negative control in the target identification. Initial inspection of cluster similarity determined by the subprofile method was inconclusive as cluster similarity was lower than 85 % (Figure S3), thus suggesting that the compounds’ mode of action differs from the activity of the ten predefined clusters.14 However, similarity to the tubulin cluster (between 76 % and 80 %, Figure S3) and the location of the compounds’ fingerprints next to the tubulin cluster in the low dimension UMAP (Uniform Manifold Approximation and Projection for Dimension reduction) plot18 (Figure 3d) suggested that the compounds might impair microtubule dynamics, mitosis and cell proliferation.16a, 19 Spirooxindoles 8 s, 8 u and 8 v inhibited proliferation of U2OS cells (Figure S4) and induced accumulation of phospho-histone H3-positive cells, indicative of mitotic arrest. The fraction of mitotic cells increased from 2 % (for the DMSO control) to 26 %, 51 % and 33 % upon treatment with 8 s, 8 u and 8 v, respectively (Figure 3e), while the structurally similar control compound 8 a was not anti-proliferative and did not induce mitotic arrest (Figure 3e and Figure S4). Impaired microtubule dynamics induces mitotic arrest, however, 8 s, 8 u and 8 v hardly inhibit in vitro tubulin polymerization at 20 μM (Figure 3f), whereas 8 s decreased polymerization by ca. 30 %.20 Thus, the compounds cause mitotic arrest by a mechanism different from tubulin modulation.
As the compounds did not display high biosimilarity to any of the predefined clusters, they most likely belong to a novel cluster. For direct identification of the cellular target, biosimilarity to fingerprints recorded for 4251 individual reference compounds (LOPAC, kinase inhibitors, Prestwick Chemical Library) was explored. Compounds 8 s, 8 u, 8 v were inactive in the CPA at 3 μM (induction <5 %), but displayed a high induction value at 10 μM (54–76 %) and decreased the cell count to 45–59 % (Figure S5). Considering the steep increase in induction and steep decrease in cell count in a narrow concentration range for compound 8 s, reference compounds most biosimilar to spirooxindole 8 s were considered irrespective of the cell count (usually, we analyze CPA fingerprints for cell counts >50 %) to avoid missing biosimilar compounds. Inhibitors of the mitotic kinesin Eg5 (ARQ-621, dimethylenastron (DME) and Ispinesib)22 and of Polo-like kinases (Ro-3280, Bl-2536, NMS-1286937)23 were among the most biosimilar compounds (see Figure 4a). Interestingly, a similar jump in induction was also characteristic for the Eg5 inhibitors ARQ-621 and DME, accompanied by a dramatic drop in cell count in a narrow concentration range (Figure S5). These findings suggest Eg5 or PLKs as potential targets.
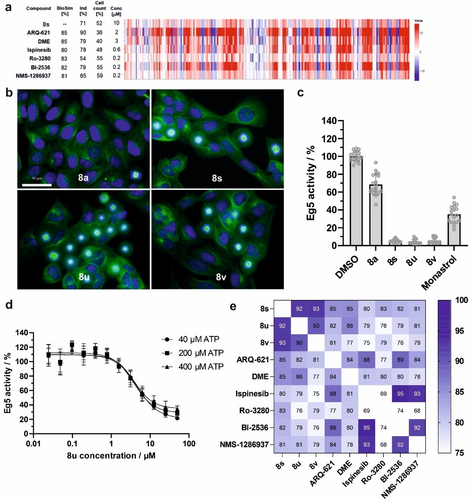
Spirooxindoles 8 s, 8 u and 8 v target Eg5. a) Eg5 inhibitors ARQ-621, Dimethylenastron (DME), Ispinesib and PLK inhibitors Ro-3280, Bl-2536, NMS-1286937 displayed high biological similarity (BioSim) with 8 s. b) Cell morphology of U2OS cells upon treatment for 24 h with spirooxindoles 8 s, 8 u and 8 v or DMSO or 8 a as controls. The cells were stained with DAPI (blue) and anti-alpha-tubulin-FITC antibody (green) to visualize the nuclei and tubulin, respectively. Scale bar: 50 μm. c) and d) Spirooxindoles 8 s, 8 u and 8 v inhibit the ATPase activity of Eg5 in the presence of microtubules. DMSO, 8 a or monastrol21 (50 μM) were used as controls. Compound concentration: 50 μM. Dose-response curve for 8 u is shown in d at different concentrations of ATP. Data are mean values ± SD of three biological replicates. e) Cross-biosimilarity of 10 μM 8 s, 8 u, 8 v, 2 μM ARQ-621, 3 μM DME, 0.6 μM Ispinesib and 0.2 μM PLK inhibitors Ro-3280, BI-2536 and NMS-1286937.
The spindle microtubule-associated ATPase motor protein Eg5 is essential for the formation of bipolar mitotic spindles during mitosis.24 Upon treatment with spirooxindoles 8 s, 8 u and 8 v but not 8 a mitotic cells exhibited exclusively monopolar spindles (Figure 4b), and the active compounds inhibited the microtubule-dependent Eg5 ATPase activity (Figure 4c).21 Spirooxindoles 8 s, 8 u and 8 v inhibited Eg5 even in the absence of microtubules (Figure S6a, b). 8 u showed similar IC50 values at different ATP concentrations, i.e. IC50 of 3.8±0.5 μM at 200 μM ATP, and of 4.6±0.5 μM and 3.9±0.4 μM at 40 and 400 μM ATP, respectively (Figure 4d). To shed light on the mode of inhibition, Michaelis–Menten kinetic studies were conducted at various concentrations of ATP and 8 u (Figure S7). Increasing the concentration of the inhibitor reduced both Vmax and Km, indicating an ATP uncompetitive mode of inhibition for 8 u. The inhibition was highly dependent on the chirality. While (R)-8 v potently inhibited Eg5 by 92 %, its enantiomer (S)-8 v showed a weak inhibition of only 33 % (Figure S6c). Furthermore, the cross biosimilarity of cell painting profiles indicated that (rac)-8 v inhibits similarly to (R)-8 v, but (S)-8 v is distinctly different from (rac)-8 v and (R)-8 v (Figure S6d), which is consistent with the chirality-dependent inhibition. The absolute stereochemistry of (R)-8 v was determined by vibrational circular dichroism (VCD) spectroscopy (see Supporting Information for details).25
PLK1 inhibition induced fingerprints analogous to the treatment with 8 s (Figure 4a). Inhibition of PLK1 affects mitotic entry, spindle assembly and cytokinesis,26 and leads to the formation of monopolar mitotic spindles.27 However, 8 s did not inhibit PLK1 and other mitosis-related kinases (Table S3), and spirooxindoles 8 s, 8 u and 8 v did not bind to the polo-box domain (PBD)28 of PLK1 (Figure S8). Thus, spirooxindoles 8 s, 8 u and 8 v target Eg5 but not PLK. Notably, Eg5 and PLK inhibitors display high fingerprint similarity (Figure 4e), such that morphological changes that are induced by them most likely define a separate bioactivity cluster and can be employed for target prediction and identification. Several Eg5 inhibitors have been reported24 and the first Eg5 inhibitor monastrol21 was identified using cell-based screening for the detection of mitosis-interfering compounds. Of note, the Eg5 inhibitor monastrol was inactive in CPA up to 50 μM which is in lines with its moderate potency (IC50=14 μM)21 and the high concentrations that are usually used in cells (>50 μM).21, 29 Although Eg5 inhibition is easily detectable using an enzymatic assay for ATPase activity or by means of DNA- and/or microtubule staining in cells, these approaches have been designed to explicitly identify Eg5 inhibitors.24 In contrast, morphological profiling aims at detecting bioactivity in an unbiased manner, thus allowing to probe a compound collection for the modulation of various targets in one single assay. Our findings add Eg5 to the list of targets that can be mapped using CPA. Of note, CPA does not use a stain for microtubules. Importantly, when considering only DNA-related features of the CPA profiles (for comparison to staining of cells for DNA and tubulin), Eg5/PLK inhibitors cannot properly be distinguished from modulators of tubulin, thus hampering precise target hypothesis generation (Figure S9a). However, the hierarchical clustering using the full profiles, i.e., considering all dye-related features, clearly distinguishes Eg5/PLK inhibitors from modulators of tubulin (Figure S9b). Thus, profiles based only on DNA-related features are not specific to Eg5 inhibitors. Hence, the full CPA profiles including features of all six dyes are required for reliable prediction of Eg5 targeting.
In conclusion, we developed an efficient scaffold-divergent synthesis of spirooxindoles through combined Lossen rearrangement and [RhCp*Cl2]2 catalyzed C−H functionalization, which is controlled by stereoelectronic effects operative in a potential rhodacycle intermediate. Morphological profiling using the Cell Painting assay directly identified Eg5 as the molecular target for spirooxindoles which defines a unique inhibitor chemotype for the kinesin Eg5.
Acknowledgments
Research at the Max Planck Institute of Molecular Physiology was supported by the Max Planck Society and the European Union (Drug Discovery Hub Dortmund, EFRE-0200481). C.G. and C.M. acknowledged the support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy (EXC-2033; project number 390677874) and the Heisenberg programme (ME 4267/5-1; project no. 418661145). We thank the Compound management and screening center (COMAS) in Dortmund for performing the Cell painting assay. The graphical abstract was generated by BioRender. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.




