Synthesis and Structural Verification of an ArI(OTf)2, NO2-Ph-I(OTf)2**
A previous version of this manuscript has been deposited on a preprint server (https://doi.org/10.26434/chemrxiv-2022-xlppr).
Abstract
PhI(OTf)2 and related ArI(OTf)2 species have been incorrectly invoked as intermediates in oxidation reactions for many years. We recently established that such compounds did not yet exist but remain an attractive target. Here we describe the synthesis, isolation, and structural characterization of NO2-PhI(OTf)2, which is resistant to decomposition and more reactive than PhI(OTf)(OAc), the species previously misidentified as PhI(OTf)2.
PhI(OTf)2 is an IIII species that was first proposed to be a discrete species by Zhdankin and co-workers in 1989.1 In the 2010s, as part of the renaissance in hypervalent iodine chemistry,2 what was purported to be PhI(OTf)2 saw increased use as a versatile oxidant in organic chemistry including synthesis of hydrazones,3 diazeniums,4 functionalized cyclopropane rings,5 the cyclization of hydroxy stilbenes or carboxylic acids,6 the functionalization of acetylenes,7 aryl C−H alkylations,8 oxyaminations,9 alpha arylations10 and oxidative coupling of NHCs.11 The most common synthetic pathway to what was thought to be PhI(OTf)2 is from commercially available PhI(OAc)2, followed by addition of 2 equivalents of TMS-OTf, typically as an in situ reaction (Scheme 1). Other than in Zhdankin's original paper indicating PhI(OTf)2 to be a reactive oil, using PhI=O and 2 TMS-OTf, no isolation has been reported. Wirth discussed spectroscopic characterization of the reaction mixture of PhI(OAc)2 and 2 TMS-OTf and concluded that 1H VT NMR experiments at −40 °C were consistent with PhI(OTf)2, although incomplete consumption of TMS-OTf is evident.9 In 2015 our group found that reaction of “PhI(OTf)2” with 2,5-diphenyltellurophene unexpectedly gave a mixed TeIV(OTf)(OAc), leading us to re-examine the reaction mixture from PhI(OAc)2 and 2 TMS-OTf and concluded that the reaction stopped at one metathesis, yielding in actual fact PhI(OAc)(OTf).12 This molecule was structurally confirmed as an isolable species by Shafir and co-workers in 2016.13 Despite these reports, descriptions of PhI(OTf)2 arising from PhI(OAc)2/2 TMS-OTf continued to appear in the literature, including invoking PhI(OTf)2 in detailed calculated reaction mechanisms/pathways.8, 10 These calculations can require a great deal of time and effort, thus it is critical the correct actual molecules are used. In 2020 we published a more detailed analysis using computational and synthetic techniques, making the conclusion that “PhI(OTf)2 does not exist (yet)”,14 including an examination of Zhdankin's original report. For Zhdankin's synthesis from PhIO and 2 TMS-OTf, we found that while the transformation was calculated to be energetically feasible with a ΔG of −41 kJ mol−1, the main observed product was the diphenyl iodonium cations, apparently arising from some electrophilic aromatic substitution (EAS) process. A phenyl containing intermediate could be detected in the 1H NMR prior to generation, but the signals were upfield from those in PhI(OAc)(OTf), and not consistent with PhI(OTf)2. For PhI(OAc)2 and 2 TMS-OTf, in addition to reconfirming the NMR data as consistent with PhI(OAc)(OTf) we calculated a ΔG for the first metathesis reaction giving PhI(OAc)(OTf) to be favourable with a ΔG of −67 kJ mol−1, but the second metathesis giving PhI(OTf)2 to be unfavourable by 30 kJ mol−1.
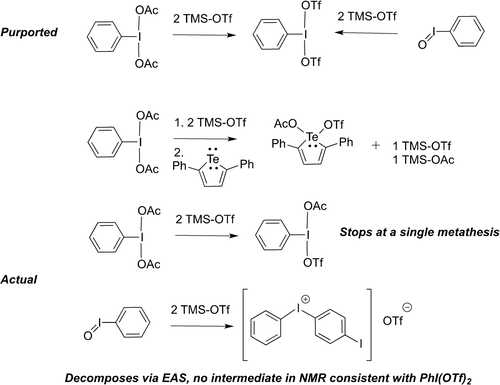
Historically claimed syntheses of PhI(OTf)2 and the findings from our lab on those syntheses.
In 2020 a study on the one and two electron oxidative capacity of PhIL2 species found that L=OTf gave by far the strongest oxidation potentials of the ligands considered making ArI(OTf)2 a highly desirable target.15 In this report we describe achieving a strategy to shut down this decomposition pathway, allowing for the synthesis, isolation and structural characterization of a genuine ArI(OTf)2.
Our strategy to achieve an ArI(OTf)2 involved a 2-pronged approach, in which we attempted to shut down decomposition by EAS (as observed via Zhdankin's pathway) and achieve a complete substitution of ligands on the iodine to triflate (which does not occur in the case of PhI(OAc)2/2 TMS-OTf). For the second goal it was decided to take advantage of the high Si−F bond strength and use ArIF2 precursors in conjunction with TMS-OTf. For suppressing EAS, we initially attempted blocking the reactive C−H positions with methyl groups. ArIF2 precursors were known for the parent phenyl, p-tolyl and 2,4,6-trimethyl derivatives. Several syntheses of these have been reported, we employed Selectfluor in conjunction with corresponding iodoarenes,16 and also used this method for the unknown pentamethyl substituted derivatives.
When PhIF2 was reacted with 2 equivalents of TMS-OTf in CD2Cl2, 19F and 1H NMR showed complete consumption of PhIF2 and the generation of a mixture, from which PhI, HF, TMS-F and unconsumed TMS-OTf could be identified, as well as other phenyl containing products. Mass spectrometry revealed the presence of iodonium cation [Ph-I-Ph-I]+ (Scheme 2), unsurprising given our previous results replicating Zhdankin's 1989 work.1, 14 Stang has reported that PhIF2 in conjunction with TMS-OTf gives a transient PhIF(OTf) species,17 which is an excellent source of the [PhI]+ cation, consistent with our observations. Blocking the most reactive para position with a methyl group and repeating the procedure gave identical results, except that the EAS reaction occurred at the ortho position of the ring. Blocking the ortho and para positions resulted in EAS at the least activated meta position of the ring. Finally, when all positions were blocked, mass spectrometry indicated the primary organic fragment to be [Ar-I-Ar]+, where a C−I bond has been apparently cleaved. This species was also present in the reaction with the tolyl derivative. We have not been able to determine a mechanism for the later transformation, but overall, this set of results indicated that blocking the C−H positions with methyl groups did not sufficiently suppress decomposition via EAS processes.
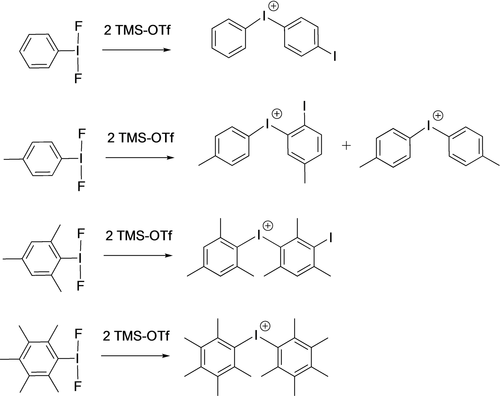
Results from TMS-OTf metathesis on methyl substituted Ar-IF2 species.
The next strategy to suppress EAS was to synthesize a derivative with a deactivating group, for which p-nitro was chosen, targeting NO2-PhIF2 as the starting material. This compound was previously reported using a 4 step synthesis involving use of gaseous Cl2 and aqueous HF.18 We accessed NO2-PhIF2 from NO2-PhI(OTFA)2, which was in turn synthesized from NO2-PhI, HOTFA and Oxone using a procedure adapted from Zhdankin.19 NO2-PhIF2 could then be easily generated via addition of Olah's reagent, pyridinium-HF in HF,20 to NO2-PhI(OTFA)2. NO2-PhIF2 presents greatly improved stability and handling properties compared to the other ArIF2 species described above, for which the syntheses are tedious, poor yielding and in our hands, readily decompose. The structure, which was previously unreported, was confirmed via X-ray crystallography. The solid is however very hygroscopic and it proved difficult to remove all traces of water, even under vacuum at 60 °C overnight, attempting to use higher temperatures started to induce decomposition. Alternatively, NO2-PhIF2 can be generated in an anhydrous manner in the glovebox from NO2-PhI and XeF2 with the reaction in CH2Cl2 initiated by catalytic TMS-OTf.
The reaction of NO2-PhIF2 (generated via Olah's reagent) with 2 equivalents of TMS-OTf in CDCl3 led to rapid dissolution of the semi-soluble NO2-PhIF2 to receive a clear solution with a yellow precipitate. In the 1H NMR spectrum 2 sets of signals consistent with NO2-Ph-X fragments were apparent. In the 19F NMR spectrum complete consumption of NO2-PhIF2 was observed with generation of TMS-F and 2 peaks in the region around −78 ppm where triflate fragments arise. Solvent removal in vacuo and reuptake of the residue in CDCl3 bared a species bearing one set of NO2-Ph-X signals and one 19F chemical shift at −75.3 ppm, consistent with covalently bound triflate, where a more ionic triflate is found up field between −78 and −79 ppm.21 The protons in the phenyl ring were 0.18 and 0.40 ppm downfield from the respective signals in the NO2-PhIF2 starting material. Single crystals were grown in the glovebox freezer via vapour diffusion at −35 °C (CH2Cl2:pentane) and X-ray studies confirmed the species as the targeted bistriflate complex NO2-PhI(OTf)2, obtainable in 92 % yield (Scheme 3). The crystals decomposed within 30 seconds of transfer from the vial into n-paratone oil at room temperature and we also experienced destroyed nylon Cryoloops during transfer from the oil onto the Goniometer head. Decomposition ceased once inside the 150 K stream of N2.
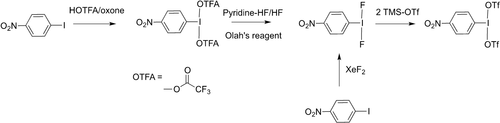
Synthetic pathway to NO2-PhI(OTf)2.
The other, CDCl3 insoluble compound precipitated in crystal quality and X-ray studies showed it to be the -NO2 analogue of Zefirov's reagent.22 We believe this arises from the residual moisture in the starting NO2-PhIF2 starting material as addition of 2 μL of water to a solution of NO2-PhI(OTf)2 in CD2Cl2 resulted in immediate conversion to the Zefirov's analogue (Scheme 4). If the NO2-PhIF2 synthesized from XeF2 is used no Zefirov's analogue is observed in the reaction with TMS-OTf.
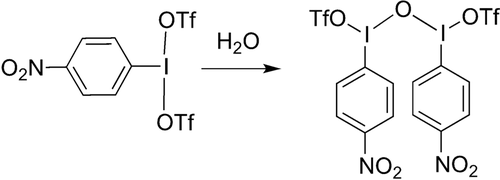
Reaction of NO2-PhI(OTf)2 with water giving the -NO2 analogue of Zefirov's reagent.
The I−O bond lengths in NO2-PhI(OTf)2 are 2.142(4) and 2.145(4) Å (Figure 1). These can be compared to the I−O bond lengths in the mixed PhI(OAc)(OTf) species determined by Shafir and co-workers which contained an I−O bond to the triflate of 2.347 Å and to the acetate of 2.056.13 This indicates a much tighter association for the triflate to the iodine in NO2-PhI(OTf)2 than PhI(OAc)(OTf); consistent with the strongly downfield 19F NMR chemical shift for NO2-PhI(OTf)2 of −75.3 ppm compared to PhI(OAc)(OTf) at −78.5 ppm.11 The strong association is also reflected in the S−O bonds, with an elongation to the O that is bound to iodine and a shortening of the other S−O bonds. The I−C bond is 2.103(5) Å, slightly elongated from 2.088 reported for PhI(OAc)(OTf). There is a long contact at 2.896 Å between the iodine and an unbound oxygen of the triflate from a neighbouring molecule in the solid state.
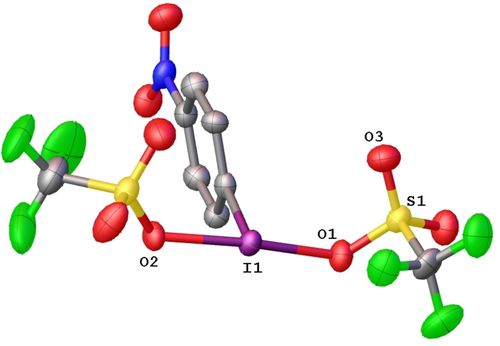
Solid state structure of NO2-PhI(OTf)2. Ellipsoids are depicted at the 50 % probability level. Selected bond lengths (Å): I(1)-O(1) 2.142(4), I(1)-O(2) 2.145(4), S(1)-O(1) 1.525(4), S(1)-O(3) 1.406(6).25
An examination of the electronic properties of NO2-PhI(OTf)2 via theoretical methods was undertaken. We had previously found that the method wPBE/def2TZVP does a better job modelling the geometry of bound triflate than other common methods,14 so this has been used here. The geometry optimization for NO2-PhI(OTf)2 closely matches the X-ray structure.
In terms of the thermodynamics of NO2-PhI(OTf)2 generation from NO2-PhIF2, using TMS-OTf, unlike PhI(OTf)2 for which the second metathesis from PhI(OTf)(OAc) is unfavourable, from NO2-PhI(OTf)2 the first metathesis to give NO2-PhIF(OTf) is favourable with a ΔG of −23 kJ mol−1 and the second giving NO2-PhI(OTf)2 is also favourable at −16 kJ mol−1. It should be noted that calculations using NO2-PhI(OAc)2 as a starting material indicate the same situation as from PhI(OAc)2, in that the first metathesis is favourable at −13 kJ mol−1, while the second is not at +23 kJ mol−1. From PhIF2, calculations indicate both metatheses are favourable with the first giving PhIF(OTf) at −34 kJ mol−1 and the second to PhI(OTf)2 at −16 kJ mol−1. Combined, these calculations together with the experimental observation of [Ph-I−Ph-I]+ with one equivalent of TMS-OTf remaining unreacted in the reaction of PhIF2 and 2 TMS-OTf, suggesting that PhIF(OTf) decomposes before reacting with the second equivalent of TMS-OTf, indicates that the -NO2 group does not provide any key driving force for the metathesis, but rather prevents the EAS decomposition pathway.
The LUMO is responsible for the electrophilic/oxidative behaviour of this class of compound. The nature and energy of the LUMO of NO2-PhI(OTf)2 was compared to known PhI(OAc)(OTf) and non-existent PhI(OTf)2 (Figure 2). The general features of the LUMO are the same in all 3 molecules, with σ antibonding character between the iodine and oxygen atoms and π antibonding character in the aryl ring. The LUMO for NO2-PhI(OTf)2 is 1.26 eV lower in energy than PhI(OAc)(OTf) and 0.48 eV lower in energy than PhI(OTf)2, suggesting incorporating the electron withdrawing -NO2 makes it a better oxidizing agent than unstable PhI(OTf)2 while shutting down the key decomposition pathway. The LUMO is more delocalized into the aryl ring and the nitro group than in the other 2 derivatives.
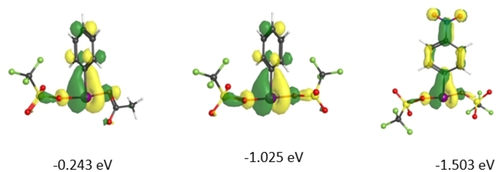
LUMOs for PhI(OTf)(OAc), PhI(OTf)2 and NO2-PhI(OTf)2.
As an initial test for the potential reactivity of NO2-PhI(OTf)2 we sought a system that was oxidizable and known to accommodate 2 triflates. Burford reported the antimony(V) compound Ph3Sb(OTf)2 as an intermediate for the synthesis of pyridine stabilized SbV dications.23 Burford accessed the compound via chloride abstraction from Ph3SbCl2, we envisioned that it could be reached via reaction of NO2-PhI(OTf)2 with Ph3Sb. The reaction was carried out in CD2Cl2. 1H NMR spectroscopy of the reaction mixture showed immediate reduction of NO2-PhI(OTf)2 into NO2-PhI and conversion of Ph3Sb into Ph3Sb(OTf)2 which was confirmed by observation of resonances identical to those reported by Burford for Ph3Sb(OTf)2 in both the 1H and 19F NMR spectra, demonstrating oxidative behaviour and delivery of both triflates from NO2-PhI(OTf)2 (Scheme 5).
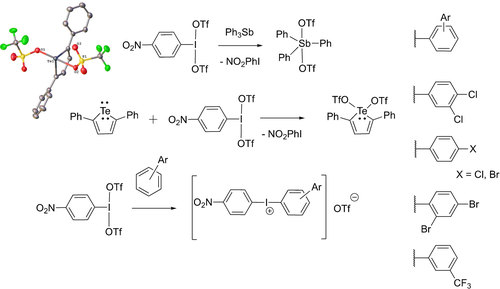
Preliminary reactivity studies on NO2-PhI(OTf)2.
With this result in hand, we targeted a less trivial example, the TeIV(OTf)2 analogue of 2,5-diphenyltellurophene, a compound we have been previously unable to synthesize via metathesis from TeIVX2 species where X=F, Cl, OAc. It has been shown the TeII/TeIV redox couple can be used to access interesting properties of tellurophene containing materials/materials precursors,24 and a bis-triflate could offer a good entry point to generating a variety of TeIV analogues by virtue of triflate being an excellent leaving group. NO2-PhI(OTf)2 was reacted with 2,5-diphenyltellurophene in CH2Cl2 solvent resulting in a deep red solution. After work-up a red crystalline material was obtained, 1H NMR in CDCl3 showed a new tellurophene species where the 1H signals in the backbone were shifted downfield 0.1 ppm from the starting tellurophene. 125Te NMR returned a chemical shift of 1024 ppm, downfield of both the TeIV bisacetate (683 ppm) and mixed acetate/triflate species (900 ppm) we have previously reported.12 The structure as the TeIV-bistriflate was confirmed by X-ray crystallography, demonstrating that NO2-PhI(OTf)2 can be used to generate previously inaccessible high oxidation state bis-triflate species.
Intermediates in attempts on the way to PhI(OTf)2 are susceptible towards EAS as a mode of decomposition as discussed above and PhI(OAc)2 that is activated by BF3 can be used as a substrate in EAS with mesitylene.13 We have found PhI(OTf)(OAc) does not react with benzene, and as a test to show the increased electrophilicity of NO2-PhI(OTf)2 it was reacted with more deactivated o-dichlorobenzene in CH2Cl2 for 30 minutes. After workup, the 1H NMR showed the formation of a product consistent with generation of an iodonium cation substituted at the 3-position. Mass spectrometry of the solution returned a spectrum dominated by a cation at m/z 393.9, consistent with (3,4-dichlorophenyl)-(4-nitrophenyl)iodonium, which was isolated in a 67 % yield. This result shows a clear increase in the reactivity of NO2-PhI(OTf)2 over PhI(OTf)(OAc), the regularly misidentified compound. Extending the scope, we found successful reactions occurred with bromobenzene, chlorobenzene, 1,3-dibromobenzene and benzotrifluoride, all relatively deactivated arenes. No reaction occurred with nitrobenzene or p-nitroiodobenzene showing there is a limit, although the no reaction with p-nitroiodobenzene is a positive feature, in that as decomposition slowly occurs NO2-PhI(OTf)2 is not then prone to react with resultant p-nitroiodobenzene.
In conclusion, a genuine ArI(OTf)2 has been synthesized. Not only can it be observed, but can be isolated, stored and structurally characterized. Its electronic properties suggest it should be a significantly more powerful oxidant than the PhI(OAc)(OTf) which has been used as “PhI(OTf)2”. Preliminary reactivity studies suggest it is indeed more reactive. We look forward to developing its chemistry and encourage others to explore its chemistry as an oxidant in inorganic and organic applications.
Our successful synthesis and isolation of a genuine ArI(OTf)2, a species we once believed to not exist, leads us to propose that deactivated nitro substituted phenyl rings could be utilised to revisit other ArI(III) species that were previously only observable or implicated by means of computation, spectroscopy, or reactivity.
Acknowledgements
We thank the ARC (FT16010007, DP200100013) and La Trobe University for their generous funding of this work. Open Access publishing facilitated by La Trobe University, as part of the Wiley - La Trobe University agreement via the Council of Australian University Librarians.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.




