LiBF4-Induced Rearrangement and Desymmetrization of a Palladium-Ligand Assembly
Abstract
Thirteen palladium-ligand assemblies with different structures and topologies were investigated for the ability to bind lithium ions. In one case, the addition of LiBF4 resulted in a profound structural rearrangement, converting a dincluclear [Pd2L4]4+ complex into a low-symmetry [Pd4L8]8+ assembly with two binding pockets for solvated LiBF4 ion pairs. The rearrangement could only be induced by Li+, indicating highly specific host–guest interactions. A structural analysis of the [Pd4L8]8+ receptor revealed a compact structure with multiple intramolecular interactions, reminiscent of what is seen for natural and synthetic foldamers.
The host–guest chemistry of palladium-based assemblies has been investigated extensively,1 and these studies have led to applications in medicinal chemistry2 and catalysis.3 The majority of the known guest molecules are either anionic4 or neutral,5 and dominant factors for guest binding are electrostatic forces and solvophobic interactions. Cations appear to be less suited as guests for PdII-based hosts, because the hosts themselves are polycationic species. However, it was found that charge-charge repulsions are not necessarily prohibitive for encapsulation of cationic guests. One possibility for diminishing detrimental Pd2+-[guest]n+ interactions is the co-complexation of anions, which act as an “electrostatic glue”.6 Other options are the functionalization of the host with cation-binding groups,7 or charge compensation by the ligand.8
We have recently observed the complexation of Na+ ions inside a tetrahedral [Pd4L8]8+ cage.6a The binding was enabled by co-encapsulation of four BF4− anions. Intrigued by the finding that a simple Na+ ion can be bound inside a [Pd4L8]8+ cage, we have examined if other [PdnL2n]2n+ cages would also be able to bind alkali metal ions. In particular, we were interested if they could bind lithium ions. Li+ is a challenging guest due to its high solvation energy.
For our investigations, we have prepared 13 different [PdnL2n]2n+ complexes (as BF4− salts), all of which had been described in the literature (Figure 1). The small library contained diverse architectures including simple dinuclear [Pd2L4]4+ complexes, a macrocyclic [Pd3L6]6+ complex, cages ([Pd4L8]8+, [Pd6L12]12+, [Pd12L24]24+), heteroleptic complexes ([Pd2L2L′2]4+ and [Pd6L6L′6]12+), and an interlocked [Pd4L8]8+ species (for details, see the Supporting Information).
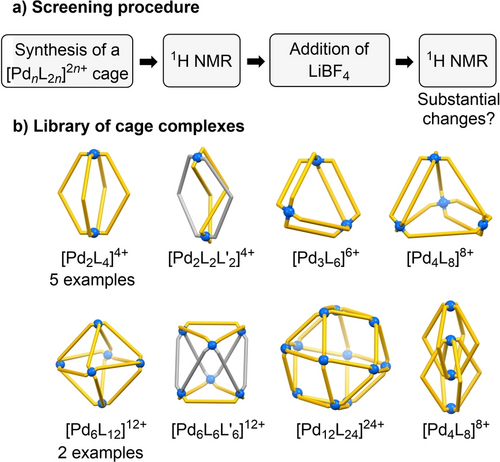
NMR-based screening for interaction of LiBF4 with [PdnL2n]2n+ assemblies (BF4− salts).
The synthesis of the 13 complexes was accomplished by mixing [Pd(CH3CN)4](BF4)2 with the corresponding ditopic N-donor ligand(s) in CD3CN and tempering for 12 h at 70 °C. The success of the self-assembly process was verified by 1H NMR spectroscopy. Subsequently, LiBF4 (50 equiv) was added and another 1H NMR spectrum was recorded. A potential interaction with LiBF4 was evaluated by comparing the spectra before and after addition of the salt. (see the Supporting Information, Figures S3–S15). The screening of 13 potential hosts gave two “hits”.
For a tetranuclear Pd complex based on ligand L1 (Scheme 1),9 the addition of LiBF4 (50 equiv) resulted in shifts in the NMR spectrum of up to 0.1 ppm (Supporting Information, Figure S3). A more elaborate NMR titration experiment was performed using a variable amount of LiBF4 (10–200 equiv, Supporting Information, Figure S16). The resulting isotherm was fitted to a 1 : 1 binding model yielding an apparent binding constant of Ka=26(±2) M−1 (Supporting Information, Figures S17).10 Li+ complexation could be corroborated by a crystallographic analysis.11 The structural data show that binding of the cation is achieved by co-encapsulation of four BF4− anions, which are situated in-between the Pd2+ ions and the Li+ ion. (Scheme 1). The larger Na+ could be encapsulated in a similar fashion, as evidenced by a crystallographic analysis of the host–guest complex (Supporting Information, Figure S35).11 A quantification of Na+ binding was hampered by the low solubility of NaBF4 in acetonitrile, which prevented NMR titration experiments.
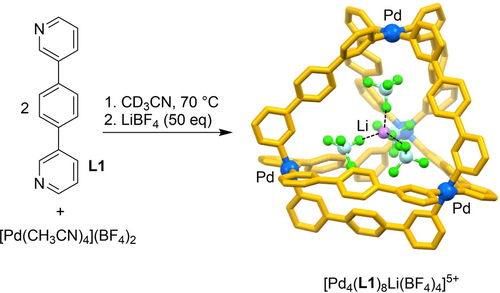
Encapsulation of [Li(BF4)4]3− by the tetranuclear complex [Pd4(L1)8]8+. The structure of the host–guest complex is based on a crystallographic analysis. Hydrogen atoms are not shown for clarity. Color coding: Pd: blue, C and N: yellow, F: green, B: light cyan.
The second “hit” in our screening was a dinuclear cage based on ligand L2.12 Directly after addition of LiBF4, we observed complexation-induced chemical shifts. However, the NMR spectrum changed with time, indicating the formation of a new complex. The rearrangement occurred with a half-life of 135 min, and it was complete within 20 hours (Supporting Information, Figures S4, S29).13, 14 Subsequent analysis by mass spectrometry revealed the formation of a [Pd4L8]8+ assembly (Supporting Information, Figures S27, S28). The tetranuclear complex could also be obtained by equilibrating a mixture of [Pd(CH3CN)4] (BF4)2, L2, and LiBF4 in a 1 : 2 : 15 ratio at 70 °C for 12 h (Supporting Information, Figure S18).
A striking feature of the new complex was its low apparent symmetry: the 1H NMR signals of the aromatic CH protons were found to split eight times (Figure 2). This splitting was evidenced by analysis of the 13C and 1H-13C HSQC NMR spectra (Supporting Information, Figures S19, S20, S22, and S23). Surprisingly, the splitting observed in the NMR spectra did not match with any of the known topologies for [Pd4L8]8+ assemblies, namely: macrocyclic,15 distorted tetrahedral,6a, 9, 16 and interlocked.5h, 17
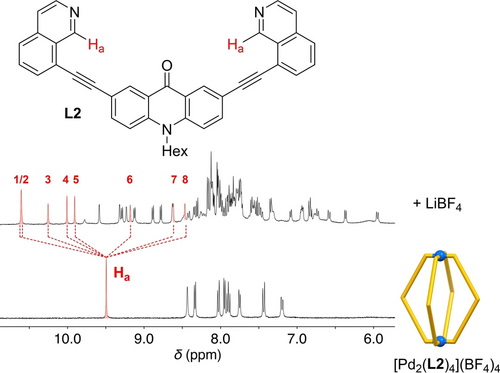
Part of the 1H NMR spectra of a mixture of [Pd(CH3CN)4](BF4)2 (1 equiv) and L2 (2 equiv) after tempering at 70 °C for 12 h in the absence (bottom) or in the presence of LiBF4 (15 equiv, top).
The rearrangement was only observed for lithium salts (LiBF4 or LiOTf). The addition of NaOTf or KPF6 to a solution of [Pd2(L2)4](BF4)4 in CD3CN resulted in broadening of the ligand signals, but a new species could not be detected. For CsBPh4, no change in the NMR spectra was observed at all (Supporting Information, Figure S32). Notably, the rearrangement was also observed for mixtures of LiOTF and NaOTf, indicating that the low-symmetry Pd complex has a very high selectivity for lithium over sodium salts (Supporting Information, Figure S33).18, 19
Single crystals of the new Pd assembly were obtained by slow vapor diffusion of diethyl ether into a solution of the complex in CD3CN.11 The results of a crystallographic analysis showed that a tetranuclear [Pd4(L2)8](BF4)8 complex had formed (Figure 3a).
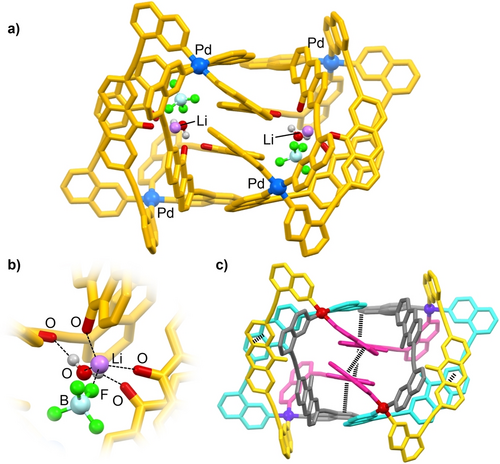
a) Molecular structure of [Pd4(L2)8]8+ in the crystal with two LiBF4⋅H2O guests molecules. Hydrogen atoms, most BF4− anions, and the hexyl side chains are not shown for clarity. Color coding: Pd: blue, O: red; C and N: yellow, F: green, B: light cyan. b) Close-up view on the binding pocket. c) Graphic representation of [Pd4(L2)8]8+ highlighting intra-ligand π-stacking interactions. The color coding indicates symmetry-related ligands and Pd2+ ions.
The assembly features two symmetry-related binding pockets (the complex has one crystallographic C2 symmetry axis).20 Each pocket is occupied by a LiBF4 ion pair and a water molecule. The Li+ ion shows a tetrahedral coordination environment with close contacts to one BF4− anion (Li−F=1.851(7) Å), one water molecule (Li−O=1.902(7) Å), and two carbonyl groups of ligand (Li−OC=1.895(8) Å, Li−O′C′=1.907(8) Å) (Figure 3b). Two other carbonyl groups are involved in hydrogen bonding to the water molecule. The latter was found to be crucial for the re-arrangement: when heat-dried LiBF4 was added to a solution of [Pd2(L2)4](BF4)2 in dry CD3CN, the formation of [Pd4(L2)8](BF4)8 could not be detected. Instead, only broadening of the 1H NMR signals was observed, similar as what was found for NaOTf. Quantitative conversion into [Pd4(L2)8](BF4)8 could then be triggered by addition of small amounts of D2O to the solution (Supporting Information, Figure S34).
The molecular structure of [Pd4(L2)8](BF4)8 in the crystal is in line with the low symmetry detected by NMR spectroscopy. There are four pairs of symmetry-related ligands (Figure 3c). Since the ligands bridge chemically non-equivalent Pd2+ ions, the two isoquinoline donor groups are also no longer equivalent.21 The four chemically distinct ligands and the reduced internal ligand symmetry gives rise to the multiplicity of 8 for the 1H NMR signals of the aromatic protons (Figure 2). The [Pd4(L2)8]8+ complex adopts a very compact structure, with numerous π-stacking interactions between the aromatic groups of the ligands (Figure 3c). Such tight intramolecular packing is reminiscent of what is found for biopolymers and for synthetic foldamers.22, 23 It is worth pointing out that the folding of [Pd4(L2)8](BF4)8 is essential for its low apparent symmetry. In terms of connectivity, the complex displays only three pairs of chemically distinct ligands (the ligands shown in Figure 3c in cyan and in grey could be interconverted by a conformational change). Attempts to unfold the assembly thermally were not successful. Even at 70 °C, the multiplicity of the NMR signals (CD3CN) was not changed (Supporting Information, Figure S26).
The prevalence of π-stacking interactions in [Pd4(L2)8]8+ suggested that hydrophobic effects might stabilize the assembly. In order to examine if a higher solvent polarity could also lead to structures other than [Pd2(L2)4]4+, we have carried out the reaction between [Pd(CH3CN)4](BF4)2 and L2 in a mixture of CD3CN and D2O (9 : 1).24 After equilibration for 5 days at 70 °C, the solution was analyzed by 1H NMR spectroscopy and mass spectrometry. The 1H NMR spectrum showed several new peaks along with those of [Pd2(L2)4](BF4)4 (Supporting Information, Figure S30), and the MS spectrum showed dominant peaks corresponding to a [Pd2(L2)5]4+ assembly (Supporting Information, Figure S31). Apparently, the increase in solvent polarity was not sufficient to promote formation of a larger tetranuclear assembly, but the results are further evidence that ligand L2 facilitates formation of alternative structures.
To conclude: Pd-based assemblies were investigated for their ability to bind lithium salts. Finding two hits within a small set of thirteen potential hosts indicates that anion-mediated binding of cations is likely a more common phenomenon for poly-cationic metal-ligand assemblies. Notably, our investigations revealed that a solvated LiBF4 ion pair can induce the rearrangement of a [Pd2L4]4+ complex into a [Pd4L8]8+ assembly. The template effect is highly specific as it requires the presence of Li+. Other alkali metal ions were not able to promote a change in structure. A unique feature of the [Pd4L8]8+ complex is its low symmetry with four chemically distinct ligand environments.25, 26 The possibility to adopt a folded, highly compact structure is a crucial factor, and ligand L2 is well suited in that regard. It displays moderate conformational flexibility, variable coordinate vectors of the N-donor groups, and large aromatic groups, which allow for π-stacking interactions. In addition, the carbonyl groups can form additional interactions with a guest. It is worth drawing a comparison with folded organic macrocycles, which were recently reported by the groups of Huc and Otto.27 Despite being obtained from only one type of building block, the macrocycles showed of up to 12 chemically distinct subcomponents. The authors argue that folding into low-symmetry structures requires building blocks with an intermediate flexibility, and with the possibility to engage in diverse non-covalent interactions. These criteria are fulfilled for L2. Current studies in our laboratory are aimed at providing more precise guidelines for the construction of homoleptic metal–ligand assemblies with folded, low-symmetry structures.
Acknowledgements
This work was supported by funding from the Ecole Polytechnique Fédérale de Lausanne (EPFL), the Swiss National Science Foundation, and by the Deutsche Forschungsgemeinschaft (DFG) through GRK2376 “Confinement-Controlled Chemistry” (Project 331085229). Open access funding provided by Ecole Polytechnique Federale de Lausanne.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.




