Relating Conformational Equilibria to Conformer-Specific Lipophilicities: New Opportunities in Drug Discovery
Abstract
Efficient drug discovery is based on a concerted effort in optimizing bioactivity and compound properties such as lipophilicity, and is guided by efficiency metrics that reflect both aspects. While conformation–activity relationships and ligand conformational control are known strategies to improve bioactivity, the use of conformer-specific lipophilicities (logp) is much less explored. Here we show how conformer-specific logp values can be obtained from knowledge of the macroscopic logP value, and of the equilibrium constants between the individual species in water and in octanol. This is illustrated with fluorinated amide rotamers, with integration of rotamer 19F NMR signals as a facile, direct method to obtain logp values. The difference between logp and logP optimization is highlighted, giving rise to a novel avenue for lipophilicity control in drug discovery.
In drug development, the focus on bioactivity has its fundamental origin in an easily understood inverse relationship between bioactivity and dose. In addition, the lower the dose, the lower the risk of toxicity and/or side effects stemming from non-specific off target activity.1 Another crucial determinant of drug dosing is lipophilicity (logP), as a proxy for a host of physical parameters related to ADMET (absorption, distribution, metabolism, excretion and toxicity).2 Optimal compound lipophilicity ranges have been proposed. It can be said that historically this aspect has been—at best—deemed secondary compared to the quest for bioactivity, resulting in worrying drug attrition rates in costly late-stage clinical trials.3 However, much effort is currently devoted to understanding structure-lipophilicity relationships including the development of tools to modify lipophilicity. Fluorine introduction is one of the possible strategies for this purpose.4 A concerted effort towards bioactivity optimization and lipophilicity control is now regarded as the best strategy for successful drug discovery.5 Novel efficiency metrics reflecting both aspects have been introduced,3, 6 and further insights and developments aiding this interactive process are of high interest.
The benefits of considering molecular conformational preferences when optimising ligand-protein interactions have been well-recognised,7 and many approaches have been studied that stabilise or even lock a flexible molecule into its bioactive conformation, in order to reduce the entropic penalty of binding. Fluorination has a notable role in this area as well.4a, 8
Lipophilicity is a molecular property (a logDpH value reflects the protonation state of ionisable species), and is defined as the concentration ratio of the solute in the octanol over the water phase ((Eq. 1), Figure 1A). This is referred to as macroscopic lipophilicity (logP). However, molecular properties result from the combination of the properties of individual conformers in solution,9 and the concept of conformer-specific partition coefficients (logp) has been introduced by Davies et al in 1979.10 These are defined as the concentration ratio of a given conformer over the two phases ((Eq. 2), Figure 1A).
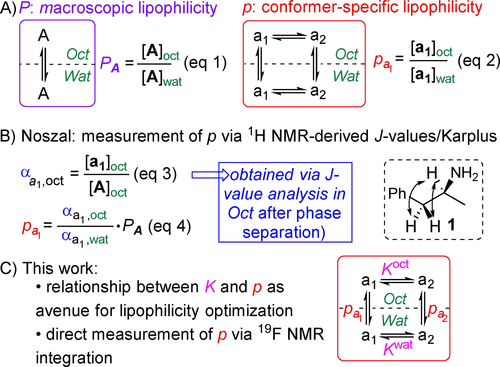
Macroscopic vs microscopic lipophilicity, measurement of the latter.
It wasn't until 2002 that the first, albeit indirect, conformer-specific lipophilicity measurements were achieved with compounds bearing vicinal J-couplings, by the group of Noszal (1, Figure 1B).11 Upon partitioning equilibration and phase separation, conformer mole fractions α (Eq. 3) in both phases were determined by NMR J-value analysis in each phase based on a Karplus analysis, allowing determination of p (Eq. 4). In this way, large conformational logp differences were found. However, there are two main problems associated with this approach. Firstly, sufficient 3J-couplings need to be measurable for unambiguous dihedral angle estimation, and secondly, J-value interpretation through the empirical Altona–Haasnoot equation is approximate.
More common is the consideration of molecular conformations for rationalisation of logP values (eg to estimate dipoles etc),12 Evaluation of calculated conformer-specific lipophilicities has been exemplified by Testa, who substantiated the “chameleonic behaviour” of morphine glucuronide: hydrophilic conformers exist in water but more lipophilic, folded conformers are adopted in lipidic media.13 The need to understand conformer specific physicochemical properties has recently gained great momentum as a strategy to obtain both water-solubility and membrane permeability of so-called “beyond Rule of 5 (bRo5)” molecules,14 and also with regard to strategies exploiting intramolecular hydrogen bonding.15 Nevertheless, these rely on calculated conformer specific properties due to the lack of experimental approaches to measure logp values, and/or on lipophilicity measurements in different solvents (eg ΔlogPoct-tol).16
Accurately calculating lipophilicities is not straightforward however. Calculated logP values obtained from 2D structures are clearly unsuitable to use for microscopic lipophilicities. For 3D structures, quantum chemistry calculations can also predict logP values, as these provide solvation energy differences between water and octanol for each conformer determining the conformer equilibria between, as well as within, the phases. However, in practice this proves difficult for systems involving small energy differences,17 while continuum solvation models also need to be applied to take into account the effect of the surroundings. Unfortunately, these implicit models are not able to take into consideration the effect of specific solvent-solute intermolecular interactions. The situation in the system under study is further complicated by the large water content in octanol, and the fact that octanol itself can engage in hydrogen bonding. Hence, the experimental determination of conformer-specific lipophilicities would be a crucial way to benchmark compounds during drug discovery optimization programs.
We report here how conformer equilibrium constants K in octanol and water are intricately linked to microscopic lipophilicities (Figure 1C), which allows logp values to be obtained indirectly. The presence of conformational equilibria distinguishes logp from logP optimization, and on this basis we propose a specific strategy towards optimizing microscopic lipophilicities. This is exemplified with a direct and convenient measurement of conformer-specific lipophilicities using our 19F NMR based logP determination procedure,18 which is possible when conformers have observable and different 19F chemical shift values. Recently, Zafrani et al. reported the logP determination of trifluoromethyl ketones and their hydrates using this NMR method, which is based on the same principle.19
The principle of the strategy for logp optimization, and of the direct logp measurement, is illustrated for amide rotamers (Figure 2) in slow exchange in solution, where each rotamer signal can be integrated directly. This constitutes a system with four coupled equilibria of conformers between (refers to p) and within (refers to K) the two phases (Figure 2A). Our lipophilicity measurement procedure18 involves acquiring a 19F NMR spectrum of each phase after phase separation (Figure 2B), and measuring integration relative to an added internal reference. Three peaks are observed: one corresponding to the reference, and two corresponding to each amide rotamer. A ρ-value for a given rotamer in a given phase is defined as the integration ratio of the rotamer signal and that of the reference (Eq. 5). Through taking the ratio of the ρ-values of a rotamer, Equation 6 can be obtained,18 showing that its logp equals the known logP of the reference plus the log of the ratio of its ρ-values. The overall logP of the amide is then determined by using the integration sum of its rotamer signals using the same equation.18
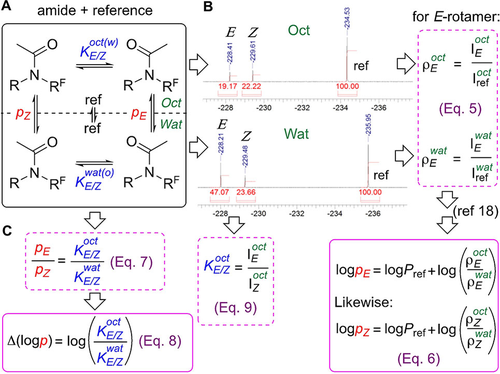
A) Equivalency between K and p-values. B) Measurement of conformer-specific lipophilicity. C) Relationship between p and K.
In the closed equilibrium system shown in Figure 2A, the ratio of the p-values equates to the ratio of the K-values ((Eq. 7), Figure 2C), so that the difference in logp values equals the log of the K-value ratio (Eq. 8). In other words: the lipophilicity difference between two conformers is directly related to the change between their equilibrium concentration ratios in water and octanol. The K-values can be obtained through integration measurement (Eq. 8). Hence, Equation 8 provides an avenue for rational logp optimization through influencing of K-values in octanol and water.
The ρ and K determinations require assignment of each fluorine signal in each phase to a particular rotamer, as it was observed that the relative position of cis-and trans-amide rotamer resonances in the 19F NMR spectrum in octanol was not always the same as that in water. Conformer signal assignment in the water phase is easily accomplished by a control experiment using (octanol-saturated) D2O by standard NOESY analysis combined with peak integration (see Supporting Information). As perdeuterated octanol is very expensive, this was experimentally non-trivial for cases when the huge octanol solvent R-CH2OH signal and its 13C satellites obscure proton resonances that are required for rotamer assignment. Solvent suppression techniques are impractical, and skew the integration ratio. We therefore introduced 1,1-dideuteriooctanol (C7H15CD2OH), which considerably facilitated rotamer assignment. It is prepared on large scale by a modified Bouveault–Blanc reduction20 of cheap methyl octanoate with EtOD as deuterium source (see Supporting Information).
With this methodology, a panel of fluorinated N-acyl piperidines and pyrrolidines as generic examples of abundant drug scaffolds21 were investigated (2–5, Figure 3A), next to a panel of 4-fluorinated prolines (6/7, Figure 3B), as specific examples where cis/trans isomerism is of particular interest.22, 23 Proline is the only amino acid for which measurable amounts of cis-amide (proline nomenclature) rotamers are observed, a behaviour which has profound biological implications.24 Wennemers et al. had shown that the rotamer ratio of 6 a/6 b is solvent-dependent,25 prompting us to investigate their logp values. As expected, the macroscopic logP of the difluorinated derivatives is larger than that of their monofluorinated counterparts for both the pyrrolidines (cf 5/4 and 3/2) and the prolines (7 a/6 a and 7 b/6 b). Additionally, for the prolines, amides b have a lower logP compared to esters a.26
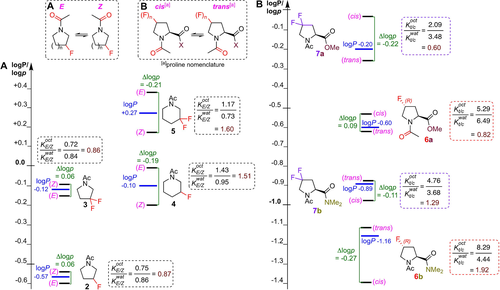
Lipophilicities (logP) and conformer-specific lipophilicities (logp) of fluorinated pyrrolidines and piperidines (A), and prolines (B).
The logp values of the 2 and 3 rotamers are similar. A gauche-effect effectively results in 2 ax as the only ring conformer (Figure 4i). While there is little difference in rotamer dipole moment, the least polar Z-rotamer turns out to be the most lipophilic. However, for 3, the Z rotamer is the most polar (not shown), while still being the most lipophilic, as the solvation energies still result in similar K-values as for 2. The gauche effect is less defining for the ring conformation of 4, with a significant amount of 4 eq in the octanol phase (Figure 4ii). Given the low dipole moment of 4 eq, its significant stabilisation in octanol results in a marked increase in  value, making the E-rotamer now the most lipophilic.
value, making the E-rotamer now the most lipophilic.
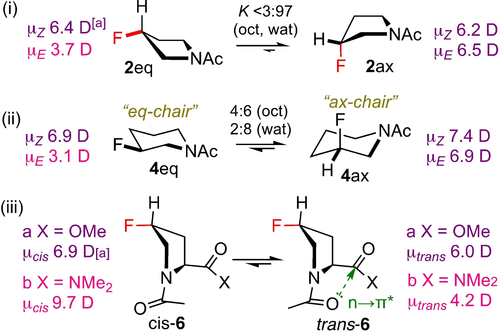
Conformations and dipole moments of selected examples. [a] Calculated in octanol at the SMD/MN15/aug-cc-pVTZ//MN15/cc-pVTZ level of theory.
For the prolines, the rotamers of 4R-4-fluoroproline methyl ester 6 a have a small logp difference, with the cis-rotamer the most lipophilic. Introducing a second fluorine (7 a) leads to a reduction in both Kt/c-values, which is more pronounced for  , hence leading to an enhanced logp difference. However, changing the ester in 6 a to the amide in 6 b enhances the
, hence leading to an enhanced logp difference. However, changing the ester in 6 a to the amide in 6 b enhances the  value, but leads to a reduction in the
value, but leads to a reduction in the  , resulting in
, resulting in 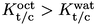 , hence the trans-rotamer is now the most lipophilic. An amide group displays increased n→π* stabilization (Figure 4iii) compared to an ester, serving to increase Kt/c. However, the much larger difference in dipole moment for the 6 b rotamers, with μcis(6 b)>μcis(6 a) (Figure 4iii) and vice versa for μtrans, results in extra stabilization of the polar cis-6 b in water, overriding the effect of the n→π* hyperconjugation, leading to a decreased
, hence the trans-rotamer is now the most lipophilic. An amide group displays increased n→π* stabilization (Figure 4iii) compared to an ester, serving to increase Kt/c. However, the much larger difference in dipole moment for the 6 b rotamers, with μcis(6 b)>μcis(6 a) (Figure 4iii) and vice versa for μtrans, results in extra stabilization of the polar cis-6 b in water, overriding the effect of the n→π* hyperconjugation, leading to a decreased  value compared to that of 6 a. Taken together, the simple modifications shown in Figure 3 clearly affect conformer equilibria, and thus conformer lipophilicities.
value compared to that of 6 a. Taken together, the simple modifications shown in Figure 3 clearly affect conformer equilibria, and thus conformer lipophilicities.
The usefulness of conformation-specific lipophilicities in a drug discovery context results from their more detailed description: instead of a one-point value (logP), a series of conformation-dependent values are obtained, the ensemble of which has been defined by Testa as “property space”.27 The physiological relevance is clear: a logP value is a population-weighted macroscopic value, while logp values can be considered being effective lipophilicities of the actual species existing in solution. This is especially of interest for so-called “sensitive molecules”27 when conformations have very different logp values. The greater this difference or “amplitude”, the greater the propensity for the molecule to adapt to its environment.28 Crucially, this may blur definitions of optimal lipophilicity ranges: while a logP value could fall outside an optimal (application-specific) lipophilicity range, one conformer, could fall within this range. For example, a molecule with a “too low” logP may have a conformer with higher logp that may facilitate membrane transport, with molecular chameleons as extreme examples.
Equation 8 (Figure 2) indicates that it is not necessary to conduct lipophilicity determinations to have information about conformer lipophilicities: knowledge of the conformer populations and K-values in each solvent will give the difference in the logp values (though not their absolute values). In this case, octanol-saturated water and water-saturated octanol would need to be used as solvents.29 Furthermore, by introducing Eqs 10 and 11, which express the relationship between logp, logP and the K values (Figure 5; see Supporting Information), absolute microscopic lipophilicity values can be obtained by measuring the K-values and the macroscopic lipophilicity P. Of course, these can also be obtained using different methods (e.g. that do not rely on the presence of fluorine, or accommodate fast exchange),30 making the approach generally applicable.
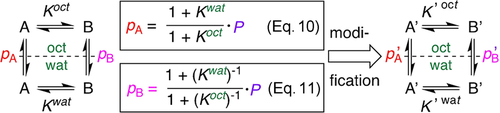
Relationship between logp and logP: the dependence on the K values. A and B are species in equilibrium. A′ and B′ arise after a structural modification.
Hence, optimization of conformer lipophilicities can be achieved by focusing on conformational equilibria: if a structural change is introduced that leads to a comparatively larger stabilization of conformer B′ in the octanol phase compared to the water phase [Δ(K′(oct)−Koct)>Δ(K′(wat)−Kwat)], then p′A<pA (over and above, but in the first instance independent from, any inherent change in macroscopic logP caused by the structural modification), and vice versa for p′B and pB. A key illustration of this is the “scaffold-hopping” from a difluorinated pyrrolidine ring to a monofluorinated piperidine such as 3→4 (Figure 3): while there is no impact on logP, the difference in K-values results in a significant impact on logp, clearly illustrating the additional information logp measurements provide. In addition, given protein binding pockets are generally more hydrophobic than the surrounding aqueous environment, optimising conformer lipophilicity values provide another useful avenue to maximising bioactive conformations. This can be illustrated with proline derivatives 7 a and 7 b, which have similar trans/cis ratios in water. However, in octanol, for the ester 7 a, this ratio decreases while for 7 b, it increases. This translates to a larger logp for the cis-rotamer for 7 a, and for the trans-rotamer in 7 b. Hence, depending on which is deemed to be the bioactive conformation, this could, in this example, guide the nature of carboxylate functionalisation before additional optimisation of the macroscopic logP.
Rational optimization requires insight in the difference in the change in K-value in water vs. octanol upon structural modification, and also how these may depend on the macroscopic logP values. Experimental logp determination in drug discovery programs will be invaluable in this regard. For the compounds reported herein, computational analysis was only moderately effective in predicting Koct/Kwat ratios (see Supporting Information).
The relationship between conformer equilibrium constant K and conformer lipophilicity logp enables a new direction towards rational lipophilicity optimization, by focusing on the difference of K values in water and in octanol upon structural modification. In addition, knowledge of logp values can guide structural modifications to optimize the lipophilicity of the bioactive conformation. The K-p relationship also shows how conformer-specific lipophilicity values can be independently obtained from the macroscopic lipophilicity and the phase-specific conformer equilibrium constants, while for specific cases involving slow chemical exchange of the species in solution, direct, straightforward measurement of conformer specific lipophilicities by our 19F NMR based method is possible. This is the first time that direct logp measurement has been demonstrated. For compounds that are in fast exchange on the NMR time-scale, conformer populations and thus conformer specific lipophilicities can be indirectly derived from NMR conformational analysis, making the approach generally applicable. In this regard, while there are many types of substrates where cis-trans isomerisms are of fundamental biological interest,22 our analysis is not restricted to conformers alone, and one can envision a more general “equilibrating species” scenario involving tautomer/ anomer equilibria, and even species in chemical equilibrium, for example hydrates,19 or any catalysed reaction, which may inspire novel thinking in property control in drug discovery. We anticipate that quantitative conformation-dependent physicochemical property measurements will inspire conformational control strategies to stabilise bioactive conformations that also shield polarity, or that are in conformational exchange equilibrium with conformations capable of shielding polarity leading to water-soluble, membrane permeable entities,31 potentially paving the way to developing 3D conformer-specific lipophilicity efficiencies. In addition, availability of experimental conformer dependent physico-chemical properties will not only help rationalise disconnections between calculated properties from 2D structures, but it will enable generation and validation of 3D based physico-chemical property models that may be impactful in the rational design of bRo5 compounds.
Acknowledgements
We are grateful to AstraZeneca for a CASE award, and to the EPSRC for a CASE Conversion, a standard, and a core capability grant (EP/M508147/1, EP/M50662X/1, EP/P019943/1, EP/K039466/1). B.L. acknowledges the Research Foundation Flanders (FWO, Belgium) for an Odysseus Type I grant (G0F5621N). Prof. Marcel Utz (University of Southampton) is thanked for insightful discussions. Dr. Neil Wells is thanked for NMR spectroscopic support. Gert-Jan Hofman and Tom Clough are thanked for providing the fluorinated prolines, and Emile Ottoy for the fluoroproline DFT calculations. Apollo Scientific is thanked for a gift of fluorinated piperidines and pyrrolidines.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




