Enantioselective Synthesis of Dithia[5]helicenes and their Postsynthetic Functionalization to Access Dithia[9]helicenes
Abstract
A highly enantioselective synthesis of 5,13-disubstituted dibenzo[d,d′]benzo[1,2-b:4,3-b′]dithiophenes is reported. Key for the successful assembly of these helical architectures is the last two successive Au-catalyzed intramolecular alkyne hydroarylation events. Specifically, the second cyclization is the enantiodetermining step of the whole process and provides the desired helicenes with excellent ee values when a TADDOL-derived 1,2,3-(triazolium)phosphonite moiety (TADDOL: α,α,α′,α′-tetraaryl-1,3-dioxolane-4,5-dimethanol) is employed as an ancillary ligand. The absolute stereochemistry of the newly prepared structures has been determined by X-ray crystallography to be P; the optical properties of these heterohelicenes are also reported. A three-step procedure was subsequently developed that allows the transformation of the initially obtained dithia[5]helicenes into dithia[9]helicenes without erosion of the enantiopurity.
Even though the chemistry of helicenes and related chiral π-conjugated structures has attracted much attention during the last few years, and a number of synthetic strategies have been developed for their preparation,1 there are still specific limitations associated with the practical assembly of these architectures. Few procedures are able to deliver the desired helicenes on a multigram scale,2 and several still rely on the traditional photocyclodehydrogenation of stilbenes, which benefits from the intrinsic preference of this reaction to deliver helical regioisomers.3, 4 In addition, synthetic routes built around highly enantioselective catalytic processes are still scarce and often have limited functional group tolerance.5 This explains why very often racemic syntheses followed by the separation of the enantiomers are employed. Furthermore, the postsynthetic C−H functionalization of already-assembled helical structures remains a challenge in terms of regioselectivity. Useful late-stage manipulations are only efficient at particularly reactive positions of (hetero)helicenes or in the presence of directing groups.6 This often forces the placement of the required functionalities at an early stage of the helicene synthesis and their transport throughout the entire sequence, either as such or in a protected form.
Considering the postfunctionalization aspect, thia[n]helicenes are arguably the most versatile structures among the helicenes, because the sulfur atom embedded in the helix offers a suitable manifold for their further diversification by directing subsequent C−H functionalizations7 or enabling the transformation of the original thiahelicene into other (hetero)helicene architecture by employing one of the aromatic metamorphosis procedures developed by Yorimitsu et al.8 Unfortunately, catalytic asymmetric routes for the synthesis of thiahelicenes are yet to be developed.9, 10, 11
During the last few years we have demonstrated the validity of the Au-catalyzed alkyne hydroarylation approach12 for the highly enantioselective assembly of [4]-, [5]-, and [6]carbohelicenes,13 as well as other chiral structures.14 This was possible only after the development of a series of chiral α-cationic phosphonite ancillary ligands derived from privileged TADDOL and BINOL platforms, which simultaneously induce enhanced reactivity to the catalyst and are able to create the appropriate chiral pocket around the linear AuI center.15
Given the electronic similarities between thiophene and benzene, we hypothesized that an extension of these synthetic strategies to the enantioselective synthesis of thiahelicenes should be possible.16 Herein, we report the practical realization of this objective through the enantioselective synthesis of dithia[5]helicenes of general formula 1. We also illustrate with a series of examples the subsequent manipulation of the initially obtained dithia[5]helicenes, either by S-oxidation or through regioselective electrophilic bromination. Finally, the dibromo derivatives thus obtained are used to expand the π-system of dithia[5]helicenes into dithia[9]helicenes through a Suzuki coupling/ Scholl cyclodehydrogenation sequence (Scheme 1).
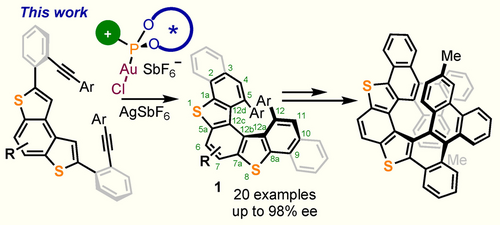
Enantioselective synthesis of 5,18-disubstituted dibenzo[d,d′]benzo [1,2-b:4,3-b′]dithiophenes and their further transformation into dithia[9]helicenes.
Our studies began with the synthesis of diyne 2 a, which was obtained by a Suzuki coupling between known 2,7-diiodobenzodithiophene 3 a and boronic acid 4 a (Scheme 2a).17 Subjecting 2 a to the action of the Au complex 5 (5 mol %), which had already been demonstrated to be one of the most efficient catalysts in our arsenal,13 afforded the desired cyclized product 1 a as a yellow solid in 81 % yield (CH2Cl2, −20 °C; 48 h). Gratifyingly, chiral HPLC analysis of that product revealed that the cyclization had occurred with remarkable enantioselectivity (92 % ee). Varying the solvent had little effect, while lowering the reaction temperature to −30 °C dramatically reduced the conversion. Switching to other α-cationic ligands did not afford better results; therefore, the already described conditions were used as standard ones to survey the scope of the reaction.
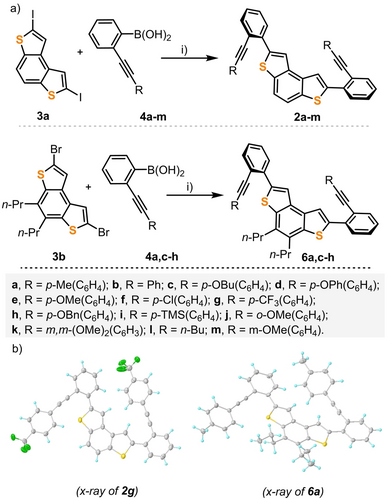
a) Synthesis of dithia[5]helicene precursors 2 and 6. Reagents and conditions: i) 3 a or 3 b (0.18 mmol), 4 a–l (3 equiv), Pd2dba3 (4 mol %), SPhos (8 mol %), and Cs2CO3 (4 equiv), THF/H2O (10 : 1, 0.02 M), 80 °C, 24 h. 2 a, 78 %; 2 b, 68 %; 2 c, 73 %; 2 d, 56 %; 2 e, 91 %; 2 f, 42 %; 2 g, 66 %; 2 h, 46 %; 2 i, 87 %; 2 j, 66 %, 2 k, 79 %, 2 l, 66 %; 2 m, 70 %; 6 a, 81 %; 6 c, 68 %; 6 d, 60 %; 6 e, 94 %; 6 f, 34 %; 6 g, 68 %; 6 h, 33 %. b) X-ray structures of 2 g and 6 a. Anisotropic displacements are shown at the 50 % probability level; the minor disorder of the solvent molecules is removed for clarity.18
Convinced by this preliminary result of the potential of our catalyst for the enantioselective synthesis of dithia[5]helicenes, additional diyne precursors 2 b–m and 6 a,c–h were prepared and screened (see the Supporting Information and Scheme 2a,b). The cyclization of diynes 2 a–i and 6 a,c–h led to 1 a–i and 1 m–s, respectively, in acceptable to excellent yields (53–98 %), while maintaining the high enantioselectivities initially observed (85–98 % ee), regardless of the electronic nature of the substituents located at the para-position of the terminal aromatic rings (Scheme 3). Halogens, ethers, trifluoromethyl, and silyl functionalities are well-tolerated. The presence of two n-propyl chains at the outer rim in 1 m–s (positions 6, and 7) hardly affects the attained ee values (compare 1 a/1 m, 1 c/1 n, 1 d/1 s, 1 e/1 o, 1 f/1 p, and 1 g/1 q). In contrast, substituents located at the meta- or ortho-position of the terminal phenyl rings drastically erode the enantioselectivity of the transformation. Thus, 1 j, 1 k, and 1 t are obtained with low or moderate ee values (45 %, 33 %, and 65 % ee, respectively). Similarly, diyne 2 l, decorated with aliphatic n-butyl groups at both alkyne termini, cyclizes into 1 l in good yield but the enantioselectivity of the process is only mediocre (60 % ee). These are the limitations of the current catalyst system, which have not been solved so far by modification of the reaction conditions or the use of another α-cationic phosphonite ligand.
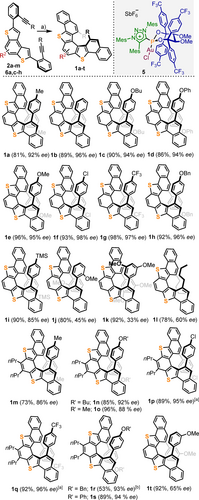
Substrate scope and limitations. Reagents and conditions: i) 5 (5 mol %), AgSbF6 (5 mol %), CH2Cl2, (0.025 M), −20 °C, 48 h. Yields are of isolated products; ee values were determined by chiral HPLC. [a] Reaction time 96 h; [b] Reaction time 72 h.
A total of seven enantioenriched dithia[5]helicenes were crystallized by slow evaporation of CH2Cl2/CH3CN (1 : 9) solutions, and the yellow crystals obtained submitted to X-ray diffraction analysis. From that series of independent measurements, the absolute configuration of these helices was unambiguously determined to be P (see Figure 1 for compounds 1 f and 1 g and the Supporting Information for the other examples). Comparison of the circular dichroism spectra (CD) of all samples permitted the generalization of this assignment to the rest of the structures (see the Supporting Information). No erosion of the enantiopurity was observed after heating a sample of 1 a (92 % ee) at 180 °C in 1,2-dichlorobenzene for 48 h, thus confirming the conformational stability imparted to the architecture by the two aromatic substituents conveniently located at the entrance of the helicene fjord.19 In addition, the synthetic practicability of the established procedure was demonstrated by the preparation of batches of 1 a and 1 e on a 150 mg scale.
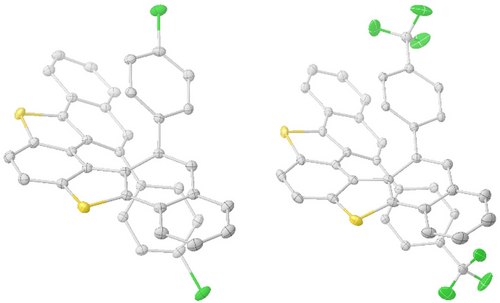
Molecular structures of compounds (P)-1 f and (P)-1 g in the solid state. Anisotropic displacements are shown at the 50 % probability level; solvent molecules and hydrogen atoms are omitted for clarity.18
The postsynthetic modification of the structures just prepared has also been approached. Treatment of the parent dithia[5]helicenes with excess (10.0 equiv) meta-chloroperbenzoic acid (m-CPBA) cleanly delivers the corresponding bis-sulfones rac-7 a and (P)-7 e in good yields (Scheme 4). It is of note that methods for the transformation of helicoidal sulfones into other (hetero)helicene architectures are already known.8 Additionally, treatment of rac-7 a with an excess of N-bromosuccinimide (NBS) (3.0 equiv) resulted in the selective dibromination of both benzylic positions to deliver rac-8 a through a Wohl–Ziegler reaction.20
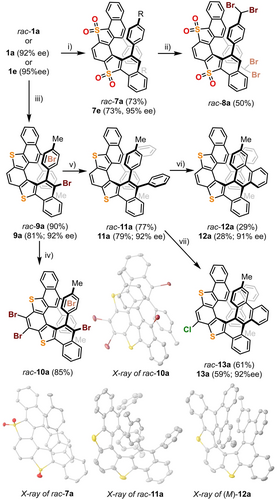
Postsynthetic manipulation of dithia[5]helicenes: synthesis of dithia[9]helicenes. Reagents and conditions: i) m-CPBA (10.0 equiv), CH2Cl2, r.t.; ii) NBS (6.0 equiv), CHCl3, reflux; iii) NBS (3.0 equiv), r.t., CHCl3; iv) NBS (3.0 equiv), CHCl3, r.t.; v) PhB(OH)2 (4.0 equiv), Pd(dppf)Cl2 (10 mol %), KF (4.0 equiv), toluene/MeOH (1 : 1), 70 °C, 9 h; vi) DDQ (5.0 equiv), MeSO3H (0.5 mL), CH2Cl2, 0 °C; vii) FeCl3 (10 equiv), CH2Cl2, r.t. Molecular structures of rac-7 a, rac-10 a, rac-11 a, and (M)-12 in the solid state. For racemic samples, only the structure of the P enantiomer is shown. Anisotropic displacements are shown at the 50 % probability level; solvent molecules and hydrogen atoms are omitted for clarity.18
Next, we aimed to evaluate the directing effect of the thienyl groups embedded in the fully aromatic helices in subsequent substitution steps. The reaction of 1 a with 3.0 equiv NBS delivers 9 a, in which the twofold bromination of the dithia[5]helicene skeleton has taken place regioselectively in excellent yield at positions 4 and 11. Further treatment of rac-9 a with an additional 6.0 equivalents of NBS cleanly affords the C2-symmetric tetrabromo derivative rac-10 a, again in good yield. These two halogenated compounds open numerous possibilities for downstream functionalization. As an illustrative example, the double Suzuki coupling reaction of 9 a with phenylboronic acid provides 11 a, which can be subsequently cyclized into 12 a through a DDQ-promoted Scholl cyclodehydrogenation reaction.21 All attempts to improve the low yield of this double cyclisation failed; however, when excess FeCl3 was employed as the oxidant, the reaction conditions could be optimized to obtain monochlorinated dithia[9]helicene 13 a as the main product.22 The transformation of 1 a into 12 a and 13 a was completed with a racemic sample for optimization purposes and subsequently with an enantioenriched one (92 % ee). No erosion of the enantiopurity has been detected along the sequence. Interestingly, racemic samples of 12 a and 13 a crystalize as mixtures of enantiopure crystals.
The UV/Vis absorption and emission spectra for selected examples (compounds 1 e, 1 g, 1 o, 12 a and 13 a) were recorded in CH2Cl2 (10−5 M) and are shown in Figure 2. The substitution pattern of the fully aromatic dithia[5]helicenes seems to have little influence on the UV/Vis spectra, which are quite similar in shape and display absorption bands up to λ=402 nm; the emission spectra are also similar and characterized by small Stokes shifts, with maxima between λ=421 and 436 nm. However, oxidation of (M)-1 e to (M)-7 e using m-CPBA results in the emission maximum being substantially red-shifted by about 80 nm, which is a clear indication of a significant perturbation of the original electronic structure. The fluorescence quantum yields are all low, but they slightly increase from Φ=0.02 in 1 e to 0.06 in 1 g, or 0.04 in 7 e (Table 1, entries 1–4). Previous reports on related structures describe a qualitatively identical behavior upon disruption of the π-conjugation by S-oxidation,23 or by the introduction of electron-withdrawing substituents at the thiahelicene skeleton, which creates a push–pull character in the structure.24 As a consequence of the increased π-extension in dithia[9]helicenes 12 a and 13 a, these compounds also show absorption maxima in the λ=410–440 nm region.
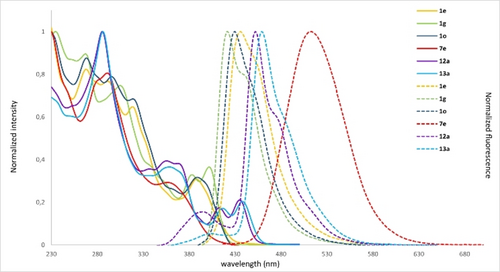
Normalized UV/Vis absorption (continuous line) and fluorescence spectra (dotted line) of 1 e, 1 g, 1 o, 7 e, 12 a, and 13 a in CH2Cl2 (10−5 M) at room temperature.
Entry |
Compound |
λabs [nm] |
logε |
λem [nm][a] |
Φf [nm][b] |
|---|---|---|---|---|---|
1 |
1 e |
268 292 318 388 |
4.69 4.66 4.59 4.27 |
436 |
0.02 |
2 |
1 g |
267 305 383 402 |
4.75 4.67 4.32 4.36 |
421 |
0.06 |
3 |
1 o |
268 297 319 389 |
4.78 4.73 4.67 4.34 |
430 |
0.03 |
4 |
7 e |
291 356 |
4.81 4.41 |
513 |
0.04 |
5 |
12 a |
285 356 413 434 |
4.93 4.53 4.18 4.28 |
394 452 |
0.02 |
6 |
13 a |
286 359 416 437 |
4.83 4.41 4.13 4.18 |
404 459 |
0.01 |
- [a] Excited at λ=310 nm. [b] Excited at λ=265 nm and using 9,10-diphenylanthracene in cyclohexane as a standard.
In summary, we report herein the first highly enantioselective synthesis of dithiahelicenes, which is achieved through a double Au-catalyzed intramolecular hydroarylation of appropriately constructed alkynes. The use of TADDOL-derived α-cationic phosphonite ancillary ligands proved to be essential to provide the appropriate chiral environment around the catalytically active Au center. Single-crystal X-ray analyses unambiguously determined the connectivity of the new structures obtained and established their absolute configuration to be P. Moreover, the regioselective postsynthetic bromination of the initially obtained dithia[5]helicene allows π-extension of that structure into dithia[9]helicenes in a short reaction sequence without loss of enantiopurity.
Acknowledgements
Financial support from the Deutsche Forschungsgemeinschaft (INST 186/1352-1 and INST 186/1237-1) is gratefully acknowledged. We also thank P. Redero for the preparation of compounds 6 h and 1 r. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.




