Light-Switchable Buffers
Abstract
A visible light-switchable buffer system based on a merocyanine photoacid is presented. Para-substitution of the indolium side with a methoxy group affords a compound suitable for making hydrolytically stable aqueous buffers whose pH can be tuned between 7 and 4 using 500 nm light.
Since the introduction of the pH scale and the word “Puffer” (in German) into the chemical lexicon by Søren P. L. Sørensen1 in 1909, buffer solutions constitute an essential tool in the study of aqueous (bio)chemical systems.2 By definition, buffers can better resist pH changes when their actual pH equals the pKa of the acid-base pair they are composed of—i.e., their buffer capacity is maximized.3 Herein, we introduce the possibility of making buffer solutions whose pH can be set at different constant values according to a light-triggered acid-base pair's pKa shift.
Protonated merocyanines (MCHs) are spiropyran compounds featuring negative photochromism.4 They are commonly referred to as “metastable-state photoacids”5—i.e., chemical species whose proton photo-dissociation is persistent enough to be coupled efficiently and reversibly with proton transfer reactions in solution.6 For this reason, MCHs are attracting increasing attention across multiple research fields spanning molecular machines,7 supramolecular chemistry,8 nanotechnology9 and materials science.10 Recently, we studied a series of MCHs bearing alkyl-1-sulfonate bridges of variable length, providing insights on their thermodynamics and kinetics in water (Figure 1 a) through cross-validation of 1H NMR, UV/Vis, and pH measurements.11 We showed that, under dark conditions, MCHs are organic weak acids displaying a ground state acidity constant close to neutrality (pKaGS=6–7), whereas, under steady light irradiation, they became more acidic by ca. 4 pK units (pKaMS=2–3).11 The extent of proton release (ΔpH) that a given MCH can achieve in solution results from a delicate interplay between its photoacidity (Π=pKaGS−pKaMS), quantum yield (Φ) and solubility, and usually lays within 1.5 pH units. Here, we expand our experimental and theoretical approaches to methoxy-substituted MCHs, dissecting the possibility of achieving reversible pH changes as high as Π, by using buffered solutions (Figure 1 b)—i.e., MCHs’ aqueous solutions whose dark equilibrium composition is not quantitatively shifted towards the undissociated form, MCH. In particular, we will show that the pH in the dark can be set at different constant values according to the Henderson-Hasselbalch equation and that ΔpH can be controlled—from 1 to 3 pH units—by carefully tuning the intensity of the light source.
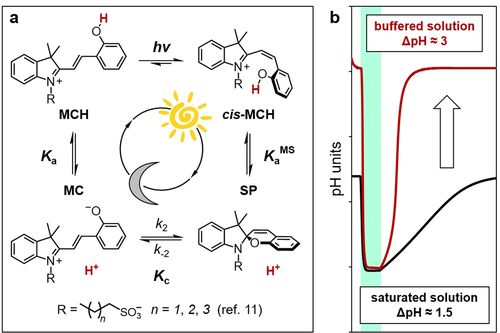
a) Four-state model describing the operation of MCHs in water. Under dark conditions, dissociation (Ka) of MCH is followed by isomerization (Kc) of MC to the corresponding SP form—i.e., KaGS= Ka(1+Kc). Under steady light irradiation, photoproduct cis-MCH undergoes ring-closing with concomitant release of a proton (KaMS). Liao's photoacid and all compounds discussed here bear a propyl-1-sulfonate group (n=2). b) Effect of buffering on 500 nm light-triggered pH jumps reported in this work.
Working with MCHs in aqueous environments can be problematic due to their tendency to hydrolyze irreversibly.12 MCHs hydrolyze with a mechanism similar to that of Schiff bases (Figure 2 a).11 We reasoned that ortho- or para-substitution of either the indolium or the chromene moiety with electron-donating groups might increase MCHs’ hydrolytic stability by disfavoring water nucleophilic attack. Therefore, we decided to quest for favorable mesomeric effects by screening four different MCHs bearing a methoxy group directly conjugated to the ene-iminium core. Compounds 1–4 were synthesized according to standard literature procedures and characterized by common techniques including X-ray crystallography13 (see SI for more details). We studied their hydrolytic stability as a function of the pH in water by UV/Vis spectroscopy, monitoring the decay of the corresponding MC(H) form at 25 °C (Figure S1). The resulting hydrolysis profiles are reported in Figure 2 b together with that (highlighted in blue) previously obtained for Liao's photoacid, which we take as reference.
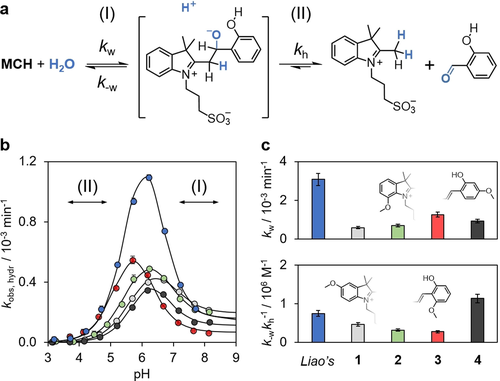
a) Mechanism of hydrolysis of Liao's photoacid below pH 9 (see ref. 11). b) Profiles of apparent first-order rate constant of hydrolysis of Liao's photoacid (blue), compound 1 (grey), 2 (green), 3 (red), and 4 (black) as a function of the pH. Solid black lines represent the best fits to Equation (S1). c) Extrapolated kinetic parameters for nucleophilic addition of water (top) and decomposition of the tetrahedral intermediate (bottom). Experimental conditions: [1–4]=25±2 μM, [phosphate buffers]=20 mM, T=25 °C.
In all cases, Equation (S1) fits the obtained bell-shaped profiles with good confidence, confirming that the rate-determining step of MCHs’ hydrolysis changes with pH from decomposition of a tetrahedral intermediate (II) to nucleophilic addition of water (I) (see SI for more details). Inspection of the obtained kinetic parameters (Figure 2 c) revealed that water nucleophilic addition proceeds most slowly in the case of 1 (grey) and that the intermediate reacts favorably—either backward or forward—only in the case of 4 (black). These preliminary findings indicate that para-substituted MCHs are better able to resist hydrolysis over the entire pH window tested. Thus, we decided to continue our investigations with compounds 114 and 412 for practical convenience.
We moved on studying the MC ⇌ SP isomerization, monitoring the decay of the corresponding MC form at 25 °C. Kinetic studies were carried out at pH 9.5, where competing hydrolytic processes are limited, and the dark equilibrium is reduced to the MC and the SP forms only. In the case of 1, we determined an isomerization constant Kc=1.46±0.07 (Figure S2). This value indicates that 1 has less tendency to equilibrate towards SP in the dark as compared to Liao's photoacid, whose Kc lays around 9±1.11 However, the rate constant of back-isomerization (k−2) was found to be similar (0.0044 s−1 vs. 0.0045 s−1), suggesting that 1 may possess comparable photochemical performance (see below). As for compound 4, the MC ⇌ SP isomerization was so fast that we were not able to determine any thermodynamic and kinetic constant under the same conditions. An alternative way to assess k−2 is to follow thermal relaxation kinetics after irradiation. The observed rate constant of relaxation in the dark matches k−2 in the pH range between pKaMS and pKa—i.e., at around pH 5 (see SI for more details).11 Therefore, we set up kinetic analyses at pH 5.2, monitoring the recovery of the corresponding MCH form at 25 °C after light irradiation (Figure S3). We found that 1 undergoes quantitative photoisomerization (>99 %) within a few milliseconds, whereas 4 isomerizes to a dramatically lesser extent (ca. 13 %). The observed rate constants of relaxation in the dark were found to be 0.0043 s−1 for 1—in good agreement with the k−2 value estimated at pH 9.5—and 4.5 s−1 for 4. Regrettably, this means that the half-life of proton photo-dissociation of compound 4 is nearly three orders of magnitude shorter than that of 1 (milliseconds vs. seconds). It follows that 4 would operate similarly to 1 only by using more intense light sources, which however we do not have. For this reason, we continued our studies focusing on compound 1.
Acid dissociation constants of 1 in the dark and under steady light irradiation were determined by UV/Vis spectroscopy, probing the dark equilibrium composition or the photostationary state at increasing pH values. According to the MC ⇌ SP isomerization studies described above, all spectra in the dark were recorded after an equilibration time of at least 15 minutes at 25 °C. In fact, only by doing so the obtained signals refer to an equilibrium situation. Under dark conditions, UV/Vis pH titrations of 1 (Figure 3 a) gave an apparent pKaGS value of 6.95±0.03. This result was confirmed by 1H-NMR analyses, which revealed that 1 features a pKa=7.4±0.1 along with a Kc=1.4±0.1, resulting in a pKaGS=7.0±0.1 (Figure S5). On the other hand, photochemical studies under steady light irradiation showed that 1 displays slightly different photostationary states depending on the wavelength of irradiation, featuring an apparent pKaMS value decreasing from 3.43±0.04 at 425 nm (Figure S6) to 3.31±0.04 at 500 nm (Figure 3 b). These studies show that the photoacidity of compound 1 (Π500=3.6±0.1) is similar to that of Liao's photoacid (Π425=3.7±0.111) but more shifted towards the neutral region by ca. 1 pK unit, and that 1 can be easily activated by exposure to visible light of longer wavelengths.
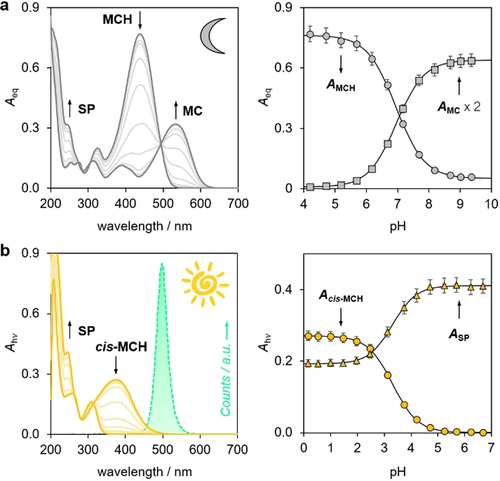
UV/Vis spectra of 1 in the dark (a) and under steady light irradiation (b) as a function of the pH. The obtained absorbance (A) profiles of MCH (437 nm), MC (534 nm), cis-MCH (375 nm), and SP (225 nm) are reported on the right. Solid black lines represent the best fit to Boltzmann's sigmoidal equation. Experimental conditions: [1]=24±2 μM, [phosphate buffers]=20 mM, T=25 °C. In the case of (b), spectra were acquired using a 500 nm LED-light source (100 mW); its emission spectrum is highlighted using the corresponding RGB color code.
We then examined the reversible proton release of compound 1 in water. pH readings at equilibrium in the dark were fully consistent with pKaGS and the total concentration of 1 in solution. Simultaneous data fitting of our four-state model to three independent variable-power pH-jump experiments carried out using either 425 or 500 nm light sources (Figures S8) revealed that the quantum yield does not change significantly by shifting the wavelength of irradiation, yielding an average value of Φ425≈Φ500=0.40±0.03. This value is close to the one obtained for Liao's photoacid by Coudret and co-workers15 (Φ436=0.38±0.03) and confirms the hypothesis that 1 does display comparable photochemical performance.
 (1)
(1)
a) Apparent solubility of compound 1 in the dark as a function of the equivalents of NaOH (α) added to the system. b) The pH of the resulting buffer solutions at equilibrium in the dark as a function of α; solid black lines represent the best fit to Equation (1) and (2), respectively. c) Reversible jumps of a solution of 1 at pH=pKaGS (α=1). d) pH tuning by modulation of the light source power. Experimental conditions: T=25 °C, 500 nm LED-light source: (c) 195 mW and (d) 40.0, 8.2 and 7.0 mW.
We ascribed the occurrence of such a linear regime to a situation where the dissociated forms 1-MC and 1-SP are effectively more soluble than 1-MCH, resulting in a Le Châtelier shift that maintains SMCH independent from α.
 (2)
(2)All in all, these observations show that the pH in the dark (and so the hydrolytic stability, see Figure 2 b) can be controlled and raised at will without diminishing the concentration of the photoactive form (1-MCH). Independent pH-jump experiments showed that ΔpH increases significantly with α, passing from 1.5 to 3.0 pH units from α=0 to α=1. Importantly, the obtained profiles of proton release/uptake were fully in line with model predictions (Figure S11). In the case of the buffer solution at pH=pKaGS we were able to trigger fully reversible jumps of 3 pH units for 2 consecutive hours without significant loss (Figure 4 c). In a separate experiment, we also demonstrated that the amplitude of the pH jump can be controlled at will by modulating the intensity of the light source (Figure 4 d).
The results presented here show that our four-state model can be extended to describe MCHs’ proton release/uptake in buffered systems. We believe our findings hold promise for implementing MCHs in the remote control of any aqueous acid-sensitive (bio)chemical system with visible light.
Acknowledgements
This work was supported by the Swiss National Science Foundation (SNSF “Ambizione” PZ00P2_180008). We thank Prof. Paolo De Los Rios, for his support and invaluable discussions.
Conflict of interest
The authors declare no conflict of interest.




