Binding of Natural Peptide Ligands to the Neuropeptide Y5 Receptor
Abstract
The binding mode of natural peptide ligands to the Y5 G protein-coupled receptor (Y5R), an attractive therapeutic target for the treatment of obesity, is largely unknown. Here, we apply complementary biochemical and computational approaches, including scanning of the receptor surface with a genetically encoded crosslinker, Ala-scanning of the ligand and double-cycle mutagenesis, to map interactions in the ligand-receptor interface and build a structural model of the NPY-Y5R complex guided by the experimental data. In the model, the carboxyl (C)-terminus of bound NPY is placed close to the extracellular loop (ECL) 3, whereas the characteristic α-helical segment of the ligand drapes over ECL1 and is tethered towards ECL2 by a hydrophobic cluster. We further show that the other two natural ligands of Y5R, peptide YY (PYY) and pancreatic polypeptide (PP) dock to the receptor in a similar pose.
Introduction
Y receptors (YRs) are a group of G protein-coupled receptors (GPCRs) phylogenetically located in the β-branch of the rhodopsin family. Four Y receptors are found in humans, Y1R, Y2R, Y4R, and Y5R, which bind neuropeptide Y (NPY), peptide YY (PYY) and pancreatic polypeptide (PP) in a partially selective fashion.1, 2 The Y1R is widely expressed both in the central nervous system and in a variety of other tissues. Y2R and Y5R are preferentially expressed in the central nervous system. The Y4R is found in the gastrointestinal tract, pancreas and prostate.1 NPY is primarily distributed in neurons and acts as a neurotransmitter, PYY is predominantly present in endocrine cells of the intestine and the pancreas, whereas PP is mainly found in pancreatic cells.3 PYY and PP act as hormones.4 NPY shares a 70 % sequence identity with PYY and a 50 % identity with PP.5 The three ligands share the common structure of a 36 amino acid chain featuring a central α-helical segment and an amidated C-terminus (Figure 1 A).1 Although Y receptors show a relatively low homology, all ligands activate all Y receptors, yet with significant differences in their activity. NPY and PYY are bound with high affinity by Y1R, Y2R and Y5R but not by Y4R. In contrast, PP binds predominantly to Y4R and with lower affinity to Y5R.6 Determinants for receptor selectivity reside in the central and N-terminal regions of the ligands, whereas the C-terminal motif (TRXRY-NH2) is crucial for receptor binding and activation.4
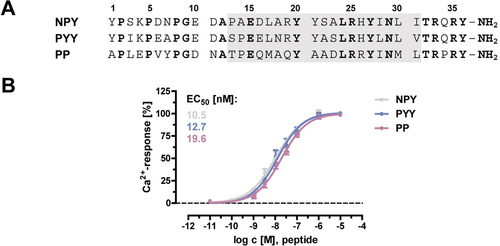
Natural ligands of the Y5R. A) Amino acid sequence of Y5R ligands. The gray box marks the conserved amphipathic α-helical segment. Conserved positions are highlighted in bold. B) Potency of Y peptides at the Y5R, dose-response curves. Data represent mean ± SEM of n ≥3 independent experiments, each performed in duplicates.
The NPY system is involved in fundamental physiological processes such as food intake, blood pressure and circadian rhythm regulation, and represents a target pursued for treatment of hypertension, epilepsy, and metabolic disorders.2, 7 Y receptors are overexpressed in many cancers and are also promising targets in cancer diagnosis and therapy.8 Substantial efforts have been devoted to developing subtype-specific ligands with therapeutic potential. However, the molecular determinants underlying receptor/ligand selectivity have been only partially identified. Only the structures of the Y1R and most recently the Y2R, both in complex with small-molecule antagonists, have been solved.9, 10 Computational models of peptide-YR complexes have been proposed, based on experimental data obtained with different approaches: the NPY-Y1R,9 the NPY-Y2R,11 and the PP-Y4R complex.12, 13 Some differences in the binding mode of Y peptides have been identified.
The Y5R shows several peculiarities within the family. Pharmacologically, it is the only Y receptor that binds all three NPY ligands with single-digit nanomolar affinity.14 It also internalizes far more slowly than the other Y receptors.15 Structurally, it has a much larger intracellular loop 3 (ICL3) (140 amino acids) and a shorter C-terminus than the others. Y5R is particularly involved in the regulation of food intake and energy homeostasis,16 as well as in the modulation of emotional processes.17 Although selective Y5R antagonists have been studied for the treatment of obesity and stress-related disorders,18 no Y5R-selective drugs have made it through clinical studies.19 Very little is known about how Y peptides bind to the Y5R. Mutagenesis studies have investigated the importance of the receptor N-terminus and a few extracellular positions.20, 21 Only very few interactions between the Y5R and its ligands have been determined.21, 22 Understanding the molecular details for binding of the natural ligands to the Y5R is an essential premise for the design of more effective and selective Y5R agents.
Here, we apply systematic photo-crosslinking mapping, alanine scanning and double-cycle mutagenesis to explore the interaction of the Y5R with its three natural ligands. Based on the experimental data, we generate a structural model of the NPY-Y5R complex, and compare determinants for the binding of PYY and PP. Our work provides the first molecular basis for the rational design of Y5R ligands.
Results
First, we mapped the footprint of NPY on Y5R by using the photo-activatable amino acid p-azido-phenylalanine (Azi) as a proximity probe (Supporting Information, Figure S1A). The Y5R was equipped with the cleavable signal peptide (SP) from influenza hemaglutinin (HA),23 which enhances its translocation to the cell membrane, and was tagged with a double HA epitope at the C-terminus. The construct SP-Y5R-2xHA exhibited a potency comparable to the wild type (wt) Y5R in a calcium flux assay when stimulated with NPY (Table 1). A tetramethylrhodamine (TAMRA) fluorophore was attached at the N-terminus of the ligand NPY by a tetraethylenglycol spacer. TAMRA-EG42-NPY behaved as a full-agonist of the Y5R and activated the receptor with acceptable potency for the purposes of photo-crosslinking (Table 1).
|
Ligand/receptor |
EC50 |
pEC50±SEM |
Emax |
Transfection |
|---|---|---|---|---|---|
|
|
[nM] |
|
[%] |
|
1 |
NPY/ Y5R |
10.5 |
8.0±0.1 |
101±4 |
stable |
2 |
TAMRA-EG42-NPY/ Y5R |
171.1 |
6.8±0.1 |
96±2 |
stable |
3 |
NPY/ Y5R |
11.0 |
8.0±0.1 |
99±2 |
transient |
4 |
NPY/ SP-Y5R-2 xHA |
27.1 |
7.6±0.2 |
80±7 |
transient |
- [a] Receptor activation was examined using a calcium flux assay in COS-7 cells expressing the Y5R and the chimeric GαΔ6qi4myr protein. Lines 1–2: Activity of modified NPY at wt Y5R was tested in COS-7 cells stably expressing Y5R-eYFP and GαΔ6qi4myr. Lines 3–4: Activation of Y5R constructs were tested in COS-7 cells transiently transfected with the receptor and GαΔ6qi4myr. Data are given as mean ± SEM of n ≥3 independent experiments.
Azi was systematically incorporated into each position of the N-terminus, the three ECLs and the upper halves of all helices of Y5R using amber suppression in HEK293T cells (Supporting Information, Figure S1B).24 Within the 122 receptor variants, Azi incorporation occurred with varying efficiency and was not tolerated only at a few sites (e.g. 2.67 and 7.34) (Figure 2 A, upper panels). Intact cells expressing each Azi-Y5R mutant were treated with TAMRA-EG42-NPY and exposed to UV light (365 nm) to trigger crosslinking. When NPY binds within about 9 Å from the Cβ of Azi,25 a covalent ligand-receptor complex is formed, which is detected in Western blot analysis by an anti-ligand antibody at the approximate molecular weight of the Y5R (Figure 2 A, lower panels). Mock transfected cells and cells expressing the wt Y5R irradiated in the presence of TAMRA-labeled NPY gave no crosslinking signals. Instead, crosslinking signals of different intensity were observed when Azi was incorporated into a subset of 25 positions, summarized in Figure 2 B and Supporting Information, Figure S1C. The N-terminus, ECL2, and ECL3 of Y5R were identified as major components of the ligand-receptor interface.
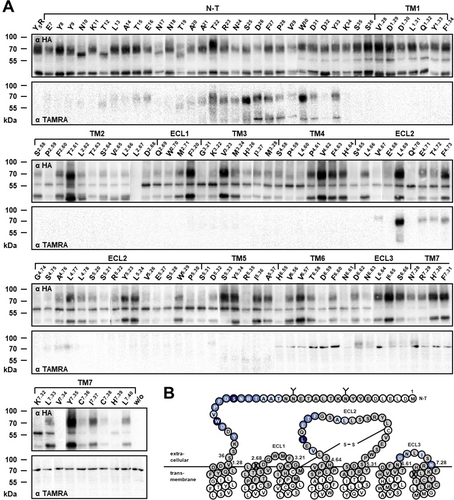
Photo-crosslinking mapping of the Y5R surface. A) Western blots of whole cell lysates resolved on SDS-PAGE and immunoblotted using both an α-HA antibody (upper panels) and an α-TAMRA antibody (lower panel). Y5R residues replaced with Azi are indicated at the top of each lane. Fully glycosylated Y5R runs at apparent MW 65–75 kDa, the non-glycosylated at 55–60 kDa. The band at 40–45 kDa could not be assigned. The covalent ligand-receptor complex runs at apparent MW 70–80 kDa. The non-crosslinked ligand (MW 5 kDa) is separated during SDS-PAGE and is not detected. Faint bands occasionally appearing at 70 kDa (for instance in the TM6-TM7) are attributable to the background of the antibody, as they are visible also in the line of non-transfected cells (last line w/o). B) Snake plot of the Y5R sequence modified after GPCRdb.org. Glycosylation sites are marked by “Y”. Residues highlighted in gray were substituted with Azi. Crosslinking hits are marked in blue. Color shades roughly reflect the crosslinking intensity. White residues were not tested. Positions that did not tolerate Azi substitution are crossed. This panel is reported in a larger scale in Supporting Information, Figure S1C.
To examine the contribution of the side chains of NPY residues to the interaction with the receptor, each of its 36 positions was substituted with alanine. Naturally occurring alanine was substituted with glycine. The potency of each Ala-NPY analogue at the Y5R was tested in a calcium flux assay (Figure 3 A and Supplementary Information, Table S1). Ala replacements in the N-terminus (residues 1–12) and in the amphipathic α-helix (residues 13–31) were in general well tolerated, whereas Ala substitutions in C-terminal positions (residues 32–36) had detrimental effects on ligand potency. Truncating the first 12 residues of NPY yielded an agonist featuring an 8-fold weaker potency (Figure 3 B). Altogether, these results identify the C-terminal segment of NPY as the driving region for Y5R activity, and suggest a minor role for the N-terminal segment.
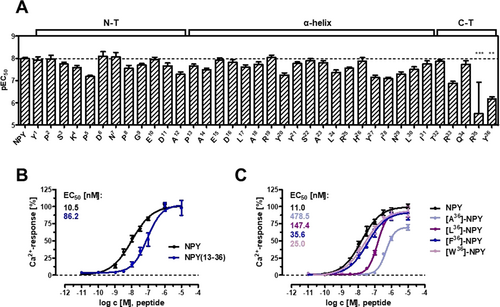
Structure–activity relationship studies. A) Alanine scan: potency of NPY analogues containing L-alanine or glycine. Bars illustrate the pEC50 values ± SEM derived from concentration-response-curves obtained by a calcium flux assay. Data are given as mean ± SEM of at least three independent experiments, each measured in duplicates. Statistical evaluation was performed using one-way ANOVA and Bonferroni's post test with ** P<0.01 and *** P<0.001 as compared to NPY. Numeric values are reported in Supplementary Table 1. B) Truncation of the N-terminal segment of the ligand and (C) substitution of Y36 to Ala, Leu, Phe, and Trp. Concentration–response curves were obtained from a calcium flux assay. Data are given as mean ± SEM of at least two independent experiments, each measured in triplicates.
We then applied double-cycle mutagenesis to pin-point intermolecular pairs of ligand-receptor amino acids involved in a direct interaction.26 The method is based on the assumption that if a distinct ligand-receptor interaction is already disrupted by a mutation in one partner (first cycle), the mutation of the counterpart in the other partner (second cycle) will have no further effects on the overall interaction. Instead, if the position mutated in the second cycle belongs to another interaction, additional effects will be observed. Based on literature data21, 22 and on homology with the NPY-Y1R complex, we proved the interaction within the NPY-Y5R amino acid pairs R25-D2.68, R33-D6.59, R35-E5.27, and L30-I6.65, and tested NPY analogues carrying Ala at position 25, 30, 33 and 35 with Y5R variants carrying Ala at residue D2.68, I6.65, D6.59 and E5.27, respectively (Figure 4). In all cases, the potency of the ligand analogues at the Ala-Y5R variant was similar to the potency of the natural ligand, which confirms the tested residues as direct counterparts in the NPY-Y5R complex. Taken together, four interactions were identified between the Y5R and NPY, which can be divided into ionic (R25-D2.68, R33-D6.59, R35-E5.27) and hydrophobic (L30-I6.65) contacts.
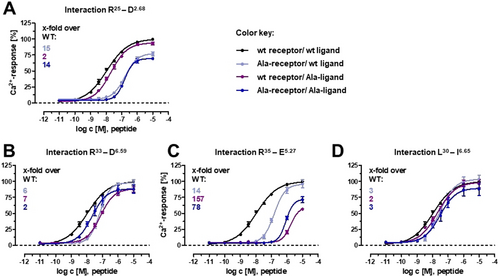
Double-cycle mutagenesis at NPY-Y5R amino acid pairs. Ligand–receptor interactions indicated in the upper line of every panel were tested. Ala mutations were introduced either in the ligand or in the receptor or in both at the putative interactivn positions. Numbers in the upper-left represent EC50 shifts relative to the wt curve (black). If the curve obtained by combining the Ala-ligand with the Ala-receptor (dark blue) shows no further right shift compared to the curve obtained when only one partner is mutated, a specific interaction between the mutated positions can be assumed. Concentration-response-curves were obtained from a calcium flux assay in transiently transfected COS-7 cells. Data represent mean ± SEM of at least three independent experiments, each performed in triplicates.
The full-length 36-mer NPY was docked into a homology model of Y5R using the software Rosetta and a hierarchical protocol mimicking conformational selection and induced fit.27, 28 Modeling of NPY into the binding pocket proceeded stepwise starting from the C-terminus, and was guided by the experimental data. Low-resolution restraints accounting for general proximity were imposed for the photo-crosslinking hits and high-resolution restraints were imposed for the proximal amino acid pairs identified by double-cycle mutagenesis (R25-D2.68, R33-D6.59, R35-E5.27, L30-I6.65).
The final structural ensemble of the ten best-scoring complex conformations is shown in Figure 5 A,B. The peptide conformation exhibits a flexible N-terminus (residues 1–12), which points away from the binding pocket. From Ala14 to Ile31 the peptide folds in an amphipathic α-helical structure, whereas the C-terminus (residues 32–36) adopts an extended conformation. NPY binds Y5R in a superficial and relatively flat pose with the C-terminal tetrapeptide penetrating into the Y5R helix bundle. The side chain of Y36 is oriented toward the center of the helix bundle and the C-terminal amide is involved in a polar contact with the side chain of Q3.32. The two arginine R35 and R33 form two salt bridges with E5.27 and D6.59, respectively, which leads the peptide from the tip of TM5 to TM6 and toward ECL3. Here, L30 and I31 form a hydrophobic cluster with L6.64 and I6.65 in ECL3. The α-helix segment of NPY is bent over the ECL1 and tethered towards ECL2 by a hydrophobic cluster formed by residue L4.69 and L5.24 with L24 and I28 and by the interaction of R25 with D2.68 on the top of TM2 (Figure 5 C,D). The N-terminal segment of the receptor (1–18) is missing in the model, due to poor homology with the template structures and lack of experimental data in this region.
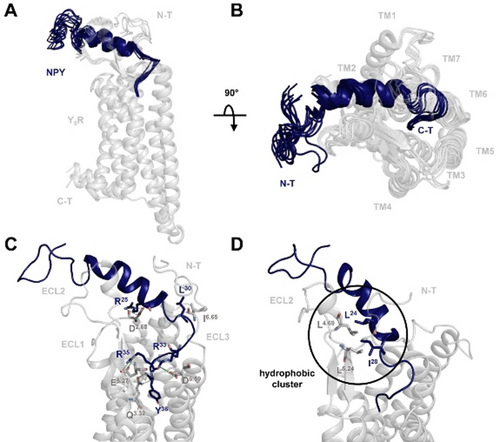
Comparative model of the NPY-Y5R complex. A) Ensemble of the lowest energy complex conformations shows a flat binding mode of the peptide (blue) to the receptor (gray). B) Top view of the predicted ligand binding mode. C) Representative side view of the complex reveals interactions of the peptide C-terminus with ECL3 and the orientation of the α-helix above ECL1. Ligand residues (blue) and receptor residues (gray) involved in ligand binding are shown as sticks. Salt bridges (green) and hydrogen bonds (orange) are shown as dashed lines. D) The NPY α-helix is bent towards ECL2 through a hydrophobic cluster between L4.69 and L5.24 with L24 and I28.
Important contacts observed in the model were validated by double-cycle mutagenesis. First, we confirmed the direct engagement of the C-terminal amide of NPY with Q3.32 in Y5R (Figure 6 A,B). Substitution Q3.32A in Y5R lead to a 3-fold loss in potency of NPY. On the other hand, NPY-tyramide, which lacks the C-terminal amide group, featured a 13-fold weaker potency compared to NPY at the wild type Y5R without further potency loss at the Q3.32A-Y5R (Figure 6 A). Likewise, a free-acid derivative of NPY lacking just the amidation (NPY-COOH) showed a 47-fold loss of potency and no additional reduction at the Q3.32A mutant (Figure 6 B). We examined then the interactions of the side chain of Y36. Strikingly, while substitution of Y36 with Ala lead to a 43-fold potency loss at wt Y5R, substitution with amino acids of increasing size and hydrophobicity (series Ala, Leu, Phe, Trp) gradually rescued the ligand activity, with W36-NPY displaying wt-like potency (2-fold loss) (Figure 3 C). On the other hand, when receptor residues lying close to Y36 (L4.60, T5.39, W6.48, F7.31, Y7.35, H7.39) were mutated to Ala, these receptor variants were activated (although in some cases with reduced potency) by NPY, but not by [Y36A]-NPY (Supplementary Table S2A highlighted lanes). Within this set, the sole combination of Ala-NPY with Ala-Y5R that gave a measurable EC50 was that of the receptor Ala-substituted at Q3.32 and [Y36A]-NPY. Taken together, these data suggest that a bulky amino acid is required at position 36 to maintain contact with several receptor residues, and that this residue may have a specific interaction with Q3.32, which is overall in agreement with the prediction of the model.
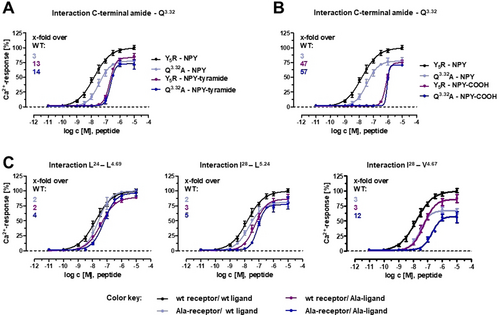
Double-cycle mutagenesis at NPY-Y5R amino acid pairs. EC50 values including confidence intervals are reported in Supplementary Table S2. A),B) NPY analogues lacking either the C-terminal CONH2 (NPY-tyramide) (A) or the C-terminal amidation (NPY-COOH free acid) feature a high loss of potency, which remains stable when mutating Q3.32 in TM3, thus validating the interaction of Q3.32 in TM3 with the C-terminal amide group. C) The hydrophobic patch between the peptide α-helix and the receptor ECL2 includes interacting residues L24 with L4.69 and I28 with L5.24, but not I28 with L4.67 (A28-NPY activates A4.67-Y5R with lower potency compared to wt NPY). Concentration-response-curves were obtained from a calcium flux assay in transient transfected COS-7 cells. Data are representative of at least two independent experiments, each measured in triplicates.
We further evaluated hydrophobic contacts predicted between the C-terminal portion of the α-helical segment of NPY and ECL2 (Figure 6 C). These include the interaction of L24 of NPY with L4.49, and the interaction of I28 of NPY with both V4.67 and the spatially close L5.24 in the antiparallel β-sheet of ECL2. The results are well compatible with interactions occurring at the two pairs L24-L4.69 and I28-L5.24, but not I28-L4.67 of Y5R.
We also investigated the role of the N-terminal segment of the Y5R, which lies close to the NPY α-helix in the model. Truncation of the first 24 or 30 residues of the receptor (Δ2-25 or Δ2-31 Y5R variants), lead to only a minor effect on receptor activation (Supplementary Information, Figure S2A).
Finally, we assessed whether the model can explain effects of variations in NPY peptides. It is known that substituting Thr32 of NPY to Pro yields a ligand that still binds Y5R but not other Y receptors.29 Indeed, we could accommodate Pro at position 32 of NPY in our NPY-Y5R model, but not into the previously published NPY-Y1R model (Supplementary Information, Figure S2D).9
After confirming that all three Y peptides, NPY, PYY and PP ligands activate Y5R with high potency (Figure 1 B), we compared the binding pocket of the three ligands.
We first screened the Y5R using Ala-mutagenesis on a set of 37 residues located within the extracellular loops and the TM helices in our Y5R homology model. The receptor was equipped with YFP at the C-terminus. Although some of these mutants showed impaired trafficking to the cell membrane (Supporting Information, Figure S3), it was possible to measure the activation of all Ala-variants by the three agonists (Figure 7 A and Supplementary Information, Table S3). Overall, the three ligands show a similar activation profile at the Ala-Y5R variants (Figure 7 B), and at the N-terminally truncated receptor (Supporting Information, Figure S2A–C). The effect of mutations at the tips of TM2, TM5 and TM6 was more pronounced for PP, whereas mutations in ECL3 had more pronounced effects on NPY.
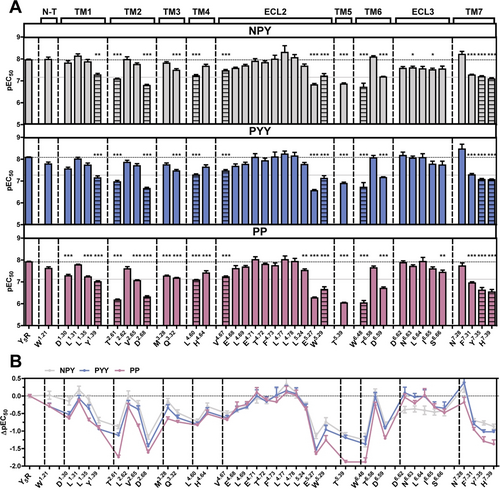
Comparison of the Y5R binding pocket between NPY, PYY and PP. A) Bars illustrate the pEC50 values ± SEM derived from concentration-response-curves obtained by a calcium flux assay in transiently transfected COS-7 cells, as given in Supplementary Table S3. The horizontal gray line indicates a 10-fold potency loss. Indicated residues were replaced with alanine. Striped bars report the results obtained with receptors that showed impaired trafficking to the cell membrane. Data are representative for at least two independent experiments, each measured in triplicates. Statistical evaluation was performed by one-way ANOVA and Bonferroni's post test with * P<0.05, ** P<0.01 and *** P<0.001 as compared to wild type Y5R. B) Differences in the impact of the examined receptor residues between the ligands displayed by ΔpEC50 values calculated from the bar graph in (A).
Furthermore, double cycle mutagenesis yielded for PYY and PP the same four spatial constraints previously determined for the NPY-Y5R interaction (Supporting Information, Figure S4 and Table S2).
The tethering of the ligand α-helix towards ECL2 through the hydrophobic interaction of residue 28 with L5.24, validated for NPY with double-cycle mutagenesis (Figure 6 C), was examined for PYY and PP. We also checked the interaction of NPY position 28 with V4.67 and investigated (at the other side of the binding pocket) the interaction of I29 of NPY with N6.63 in ECL3 (Supporting Information, Figure S5). Patterns observed for position 28 of PYY (Leu) and PP (Ile) are similar to those observed with NPY (Ile) and support a contact with L5.25 but not V4.47. Considerable differences were instead observed at position 29 (Asn, conserved in all three ligands). Ala at this position reduces the potency of PYY and PP at the wt Y5R, but not the potency of NPY. No further potency loss was observed at the A6.63-Y5R, which corroborates the N29-N6.63 interactions with PYY and PP.
With respect to C-terminal residue of the ligand, mutations of putatively interacting residues of the receptor, as well as replacement of Y36 with Ala, yielded for PYY an PP the same pattern observed at NPY (Supporting Information, Table S2B,C), suggesting that Y36 is similarly orientated inside the helix bundle.
Finally, we examined a few positions in the C-terminal segments of the peptides using Ala analogues. We chose R35 and Y36, which displayed the strongest impact in NPY (Figure 3 A), Y27, which was previously proposed to be essential for Y5R binding21 and H/R26, which is not conserved. All R35A analogues display a greater than 100-fold loss of potency, which is the largest effect of all tested analogues and supports the importance of the positively charged residue for ligand recognition (Figure 8 and Supporting Information, Table S4). Substitution of Y36 to alanine yielded a greater than 40-fold increase in the EC50 value for all the peptides. Differences among NPY, PYY and PP were found for position 26 and 27. Exchange of His26 of NPY or PYY did not much affect the activity of the agonists (1-fold and 2-fold loss, respectively), whereas replacement of Arg26 of PP induced a 5-fold activity loss. By contrast, substitution of position 27 seriously influenced the potency of NPY (7-fold loss) and PYY (10-fold loss), but not that of PP (2-fold loss). These results suggest that, while the receptor distinguishes between position 26 and 27 among the ligands, there is no discrimination at positions 35 and 36.
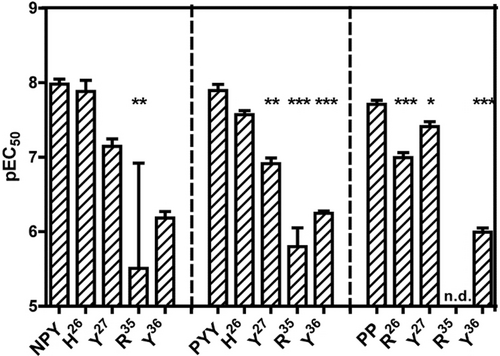
Comparison of position 26, 27, 35, and 36 between NPY, PYY and PP. Bars illustrate the pEC50 values ± SEM derived from concentration-response-curves obtained from a calcium flux assay performed in stably transfected COS-7-Y5R-eYFP-GαΔ6qi4myr. Data are representative of n≥2 independent experiments, each measured in duplicates. Statistical evaluation was performed using one-way ANOVA and Bonferroni's post-test with * P<0.05, ** P<0.01 and *** P<0.001 as compared to the wild type ligand. n.d.- not determinable.
Discussion
The Y system, composed in humans by four GPCRs and three peptide ligands, is involved in regulating crucial physiological functions and represents an important target for medical intervention. As natural Y peptides interact with all Y receptors, the challenge is to develop subtype selective ligands. In this context, it is essential to understand how natural ligands bind and activate each Y receptor. In the lack of 3D structural data for any peptide complex with a Y receptor, computational models based on experimental constraints provide the best possible approximation. While such models have already been published for Y1, Y2, Y4, the first detailed molecular model for the NPY-Y5R interaction is presented here. The model is based on data from extensive photo-crosslinking mapping and mutagenesis studies carried out on the fully glycosylated receptor at the surface of the live cells.
A first overview of the binding pocket of Y5R for NPY was gained by mapping the whole juxtamembrane region with a photo-crosslinker, the non-canonical amino acid Azi. Genetically encoded crosslinkers30 have been widely applied to investigate peptide-GPCR interactions31 both at rhodopsin-like32, 33 and secretin-like GPCRs.25, 34 Most recently, the method has been expanded to study GPCR-arrestin interactions35 and GPCR oligomerization.36 Photo-crosslinking mapping of Y5R with Azi yielded crosslinking hits in three well-defined regions of the receptor: The N-terminus, ECL2, and ECL3.
In the N-terminus, Azi captured NPY from almost all sites between A21 and Y33. Our model predicts that this segment is close to the C-terminal portion of the α-helical segment of NPY. The N-terminus of rhodopsin-like GPCRs is often short and unstructured, but it may play an important role in ligand binding, especially for peptide ligands, as it has been shown at the neurokinin-1 receptor.32 The homogeneous distribution of crosslinking hits speaks for an unstructured and highly dynamic conformation of this receptor region. As truncation of the first Y5R 30 residues did not significantly affect receptor activation by any of the three natural ligands, no specific interaction with the ligand is suggested.
The second photo-crosslinking region is ECL2, with hits distributed across the stretch V4.07-L4.77. Notably, Azi incorporation was poorly tolerated in this loop, a fact that has been observed also with other GPCRs.25, 32 In our model, secondary structural restraints were placed on ECL2 to ensure the antiparallel β-strand conformation, which is typical for peptide-activated GPCRs.28 ECL2 lies almost parallel to the ligand and forms with it a cluster of hydrophobic interactions, which were validated by mutagenesis and resemble the hydrophobic patch predicted between NPY and the ECL2 of Y2R (L24 and I28 with I4.71 and I4.77).11 The ECL2 was also reported to form part of the ligand binding pocket of Y1R.9 In general, ECL2 is known to be relevant for ligand recognition and signaling in several GPCRs.37
Finally, crosslinking hits were observed in ECL3, although the intensity was overall lower compared to the other two regions. Still, the model predicts a hydrophobic cluster between NPY and Y5R. Interestingly, ECL3 is not crucial for ligand binding at Y2R and Y4R, whereas it has been reported to be important for Y1R.9
Overall, the lack of crosslinking hits in deep regions of the TM helices suggests NPY achieves a quite superficial pose within the binding pocket of Y5R, which is well reflected in the model. The NPY-Y5R interaction involves several electrostatic interactions, including the three proximal amino acid pairs identified with double-cycle mutagenesis (R25–D2.68, R33–D6.59, R35–E5.27). Those interactions are in close agreement with the model suggested for the Y2R,11 and match well the observation that the NPY-Y5R attraction is dramatically reduced in the presence of divalent ions.21
From the perspective of the ligand, the results of the Ala scan identify the C-terminal segment of NPY, especially the last four residues, as determinant for Y5R activation, in line with what has been observed for all other YR subtypes.38 By substituting Y36 with amino acids of increasing size, we reveal that the presence of a bulky amino acid at the C-terminus is particularly important. In the model, this bulky side chain dives into the TMD between TM3, 5, and 6 and engages in an extended interaction network with the receptor. Mutagenesis of the receptor shows that none of these interactions is essential for activation. Therefore, we suggest that the conformational rearrangement induced by the binding of the bulky moiety triggers the outward movement of TM6, which is the main hallmark of activation for all GPCRs of all classes.39 On the basis of our model and the mutagenesis data, we further propose that the C-terminal amide of NPY interacts with Q3.32 in TM3 of Y5R, as observed in the homologous NPY-Y2R model.11 Q3.32 is highly conserved among the YRs and this interaction likely represents a hallmark for the entire multiligand/multireceptor system.21
With respect to the N-terminus of the ligand, our model does not predict direct interactions with the Y5R, similar to the NPY-Y2R and PP-Y4R models,11-13 whereas the N-terminus of NPY was suggested to interact extensively with Y1R.9 Indeed, N-terminally truncated NPY can bind Y2R but not Y1R.40 Interestingly, while Ala substitution the N-terminus of NPY barely affected Y5R activation, truncation of the 12 N-terminal residues reduced the potency of the ligand by 8-fold. As altering the structure of the N-terminal region of NPY can heavily affect ligand potency even at YR subtypes that tolerate its truncation,41 the N-terminal segment may stabilize the overall conformation of the peptide.
Overall, the binding mode of NPY to Y5R shows the greatest similarity to the NPY binding at Y2R, with the Y5R ECL2 interacting with the NPY α-helix, while the N-termini of both peptide and receptor have a minor relevance.11 In comparison, both the receptor N-terminus and the ECL2 are essential in recognizing and binding NPY to Y1R,9 while neither domain is required for PP binding to the Y4R. Apparently, the N-terminus and the ECL2, which are the least conserved extracellular regions among the YRs, confer the ligand specificity within the multiligand/multireceptor system. Our NPY-Y5R model allows furthermore to rationalize the experimental finding that the T32P-NPY analogues is a selective Y5R agonist, as this Pro substitution is energetically accepted in NPY docked to Y5R but not to Y1R (Supporting Information, Figure S2D).
Y5R is the only Y receptor that is activated by all three Y ligands with about the same potency. Physiologically, only the interaction with NPY and PYY may be relevant, being both ligands expressed in the same regions of the central nervous system as the receptor,1, 4 whereas PP is expressed in the pancreas along with no Y5R expression. Our results suggest that the three ligands have quite similar binding patterns, with extended overlapping regions. A main difference between PP and the other two ligands was found in the divergent position 26 (His in NPY and PYY, Arg in PP) and at Y27, which show strikingly different tolerance toward Ala substitution. In our model, Y27 (conserved) points toward the cytosol and interacts with the N-terminus of the Y5R, whereas H26 points toward TM2, tethered in this pose by the experimental constraint D2.68-R25 (conserved). The data imply a different pose of these residues of PP in this region of the binding pocket.
Conclusion
We have unveiled how the neuropeptide NPY binds to the Y5R, thus filling in the gap in the knowledge of the Y system. Although Y5R antagonists have been mainly developed to inhibit food intake, Y5R has recently been shown to also play essential roles in stress resilience, membrane excitability, and liver cancer.42 The knowledge of the binding site of the peptide agonist puts the basis for the design and the development of agonists that can selectively target only specific Y5R's functions of interest.
Acknowledgements
We thank Kristin Löbner for excellent technical assistance in cell culture, Janet Schwesinger, Christina Dammann, and Regina Reppich-Sacher for technical assistance in sequencing and mass spectrometry, and Ronny Müller for automated peptide synthesis. S.R. is grateful to Dr. Lisa Seidel for her excellent advice on the photo-crosslinking technique and Dr. Tobias Fischer for help with editing. We acknowledge funding by the German Research Foundation (DFG) through CRC 1423, project number 421152132, subproject B04 to I.C., B01/Z03 to A.B.S. and A07/Z04 to J.M. J.M. is further supported by NIH NIDA R01 DA046138. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.




